![]() DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO, Novel Drug Approvals for 2017, A Review Compilation (USFDA, EMA).
DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO, Novel Drug Approvals for 2017, A Review Compilation (USFDA, EMA).
Any errors in this compilation, email amcrasto@gmail.com, Call +919323115463
Some gaps will be filled up soon keep watching……………..
INDEX, NAME (click on the title, it contains link)
SECTION A; USFDA Approvals
6 BENRALIZUMAB
17 DURVALUMAB
24 GUSELKUMAB
36 OZENOXACIN
40 SARILUMAB
41 SECNIDAZOLE
INDEX, FORMULATION NAME
USFDA
•Aliqopa (COPANLISIB) to treat adults with relapsed follicular lymphoma — a slow-growing type of nonHodgkin lymphoma (a cancer of the lymph system) — who have received at least two prior systemic therapies;
• ALUNBRIG, BRIGATINIB, To treat patients with anaplastic lymphoma kinase (ALK)-positive metastatic non-small cell lung cancer (NSCLC) who have progressed on or are intolerant to crizotinib
• Austedo, Deutetrabenazine For the treatment of chorea associated with Huntington’s disease
• Bavencio (avelumab) for the treatment of patients 12 years and older with a rare and aggressive form of cancer called metastatic Merkel cell carcinoma, including those who have not received prior chemotherapy;
•BAXDELLA, Delafloxacin, BACTERIAL INFECTIONS
• Benznidazole to treat children ages 2 to 12 years with Chagas disease, a parasitic infection that can cause serious heart illness after years of infection, and can also affect swallowing and digestion. This is the first treatment approved in the United States for this rare disease;
• Besponsa (inotuzumab ozogamicin) for the treatment of adults with a type of cancer of the blood called relapsed or refractory B-cell precursor acute lymphoblastic leukemia;
•BEVYXXA, BETRIXABAN, For the prophylaxis of venous thromboembolism (VTE) in adult patients hospitalized for an acute medical illness
• BRINEURA, CERLIPONASE ALFA, To treat a specific form of Batten disease
• Calquence (ACALABRUTINIB) to treat adults with mantle cell lymphoma who have received at least one prior therapy. Mantle cell lymphoma is a particularly aggressive cancer;
• DUPIXENT, (DUPILUMAB) To treat adults with moderate-to-severe eczema (atopic dermatitis)
• Emflaza (deflazacort) to treat patients age 5 years and older with Duchenne muscular dystrophy, a rare genetic disorder that causes progressive muscle deterioration and weakness;
• FASENRA, BENRALIZUMAB, For add-on maintenance treatment of patients with severe asthma aged 12 years and older, and with an eosinophilic phenotype
• Giapreza (angiotensin II), for the treatment of hypotension in adults with distributive or vasodilatory shock (dangerously low blood pressure despite adequate heart function) whose blood pressure remains low despite receiving fluids and treatment with drugs called vasopressors;
• HEMLIBRA EMICIZUMAB To prevent or reduce the frequency of bleeding episodes in adult and pediatric patients with hemophilia A who have developed antibodies called Factor VIII (FVIII) inhibitors.
• Idhifa (enasidenib) for the treatment of adults with relapsed or refractory acute myeloid leukemia, a form of blood cancer, who have a specific genetic mutation;
• IMFINZI, DURVALUMAB To treat patients with locally advanced or metastatic urothelial carcinoma
• Ingrezza (valbenazine) to treat adults with tardive dyskinesia, a side effect of some antipsychotic medications whereby patients can experience uncontrollable stiff, jerky movements of their face and body, and other uncontrolled movements such as eye-blinking, sticking out the tongue, and arm-waving;
• KEVZARA SARILUMAB, RHEUMATOID ARTHRITIS
• KISQALI, RIBOCICLIB, To treat postmenopausal women with a type of advanced breast cancer
• Macrilen macimorelin acetate, For the diagnosis of adult growth hormone deficiency
• Mavyret (glecaprevir and pibrentasvir) to treat adults with chronic hepatitis C virus genotypes 1-6 without cirrhosis (liver disease) or with mild cirrhosis, including patients with moderate to severe kidney disease, as well as those who are on hemodialysis;
• Mepsevii (vestronidase alfa-vjbk) to treat patients with Sly syndrome or mucopolysaccharidosis type 7 – a rare genetic disorder where an enzyme deficiency results in skeletal abnormalities, developmental delay, enlarged liver and spleen, and narrowed airways, which can lead to respiratory infections;
• Nerlynx (neratinib) for the extended adjuvant treatment — a form of therapy administered after an initial treatment to further lower the risk of the cancer coming back — of early-stage, human epidermal growth factor receptor 2 (HER2)-positive breast cancer;
• OCREVUS, OCRELIZUMAB, To treat patients with relapsing and primary progressive forms of multiple sclerosis
• OZEMPIC SEMAGLUTIDE To improve glycemic control in adults with type 2 diabetes mellitus
•PARSABIV, ETELCALCETIDE, To treat secondary hyperparathyroidism in adult patients with chronic kidney disease undergoing dialysis
• Prevymis (letermovir) for prevention of an infection called cytomegalovirus (CMV) in patients who are receiving a bone marrow transplant. CMV disease can cause serious health issues in these patients;
• Radicava (edaravone) to treat patients with amyotrophic lateral sclerosis, commonly referred to as Lou Gehrig’s disease, a rare disease that attacks and kills the nerve cells that control voluntary muscles;
• RHOPRESSA, NETARSUDIL, To treat glaucoma or ocular hypertension
• Rydapt (midostaurin) to treat adults newly diagnosed with a form of blood cancer known as acute myeloid leukemia who have a specific genetic mutation called FLT3, in combination with chemotherapy;
• Siliq (brodalumab) to treat adults with moderate-to-severe plaque psoriasis, a chronic disorder in which the body’s immune system sends out faulty signals that speed growth of skin cells that then accumulate, causing red, flaky patches that can appear anywhere on the body;
•SOLOSEC, SECNIDAZOLE To treat bacterial vaginosis
• STEGLATRO ERTUGLIFLOZIN To improve glycemic control in adults with type 2 diabetes mellitus
• Symproic (Naldemedine) for the treatment of opioid-induced constipation in adults with chronic noncancer pain; • Tremfya (guselkumab) for the treatment of adults with moderate-to-severe plaque psoriasis;
• Trulance (plecanatide) to treat adults with chronic idiopathic constipation, which is a persistent condition of constipation due to unknown origin;
• TYMLOS, Abaloparatide, To treat osteoporosis in postmenopausal women at high risk of fracture or those who have failed other therapies
• Vabomere (vaborbactam and meropenem) for treatment of adults with complicated urinary tract infections, including pyelonephritis (kidney infection) caused by bacteria;
• Verzenio (abemaciclib) to treat adults who have hormone receptor (HR)-positive, HER2-negative advanced or metastatic breast cancer that has progressed after taking therapy that alters a patient’s hormones (endocrine therapy);
• Vosevi (sofosbuvir/velpatasvir/voxilaprevir) to treat adults with chronic hepatitis C virus genotypes 1-6 without cirrhosis (liver disease) or with mild cirrhosis;
• Xadago (safinamide) as an add-on treatment for patients with Parkinson’s disease who are currently taking levodopa/carbidopa and experiencing “off” episodes;
• XERMELO, TELOTRISTAT ETHYL combined with somatostatin analog (SSA) therapy to treat adults with carcinoid syndrome diarrhea that SSA therapy alone has inadequately controlled, and;
• XEPI OZENOXACIN TO TREAT IMPETIGO
•XERMELO, TELOTRISTAT ETHYL, To treat carcinoid syndrome diarrhea
• Zejula (niraparib) for the maintenance treatment (intended to delay cancer growth) of adults with recurrent epithelial ovarian, fallopian tube or primary peritoneal cancer, whose tumors have completely or partially shrunk (complete or partial response, respectively) in response to platinum-based chemotherapy
USFDA
| No. | Drug Name |
Active Ingredient | Approval Date | FDA-approved use on approval date |
|---|---|---|---|---|
| 46. | Giapreza | angiotensin II | 12/21/2017 | To increase blood pressure in adults with septic or other distributive shock Press Release Drug Trials Snapshot |
| 45. | Macrilen | macimorelin acetate | 12/20/2017 | For the diagnosis of adult growth hormone deficiency Drug Trials Snapshot |
| 44. | Steglatro | ertugliflozin | 12/19/2017 | To improve glycemic control in adults with type 2 diabetes mellitus Drug Trials Snapshot |
| 43. | Rhopressa | netarsudil | 12/18/2017 | To treat glaucoma or ocular hypertension Drug Trials Snapshot |
| 42. | Xepi | ozenoxacin | 12/11/2017 | To treat impetigo Drug Trials Snapshot |
| 41. | Ozempic | semaglutide | 12/5/2017 | To improve glycemic control in adults with type 2 diabetes mellitus Drug Trials Snapshot |
| 40. | Hemlibra | emicizumab | 11/16/2017 | To prevent or reduce the frequency of bleeding episodes in adult and pediatric patients with hemophilia A who have developed antibodies called Factor VIII (FVIII) inhibitors. Press Release Drug Trials Snapshot |
| 39. | Mepsevii | vestronidase alfa-vjbk | 11/15/2017 | To treat pediatric and adult patients with an inherited metabolic condition called mucopolysaccharidosis type VII (MPS VII), also known as Sly syndrome. Press Release Drug Trials Snapshot |
| 38. | Fasenra | benralizumab | 11/14/2017 | For add-on maintenance treatment of patients with severe asthma aged 12 years and older, and with an eosinophilic phenotype Drug Trials Snapshot |
| 37. | Prevymis | letermovir | 11/8/2017 | To prevent infection after bone marrow transplant Drug Trials Snapshot |
| 36. | Vyzulta | latanoprostene bunod ophthalmic solution | 11/2/2017 | To treat intraocular pressure in patients with open-angle glaucoma or ocular hypertension. Drug Trials Snapshot |
| 35. | Calquence | acalabrutinib | 10/31/2017 | To treat adults with mantle cell lymphoma Press Release Drug Trials Snapshot |
| 34. | Verzenio | abemaciclib | 9/28/2017 | To treat certain advanced or metastatic breast cancers Press Release Drug Trials Snapshot |
| 33. | Solosec | secnidazole | 9/15/2017 | To treat bacterial vaginosis Drug Trials Snapshot |
| 32. | Aliqopa | copanlisib | 9/14/2017 | To treat adults with relapsed follicular lymphoma Press Release Drug Trials Snapshot |
| 31. | benznidazole | benznidazole | 8/29/2017 | To treat children ages 2 to 12 years old with Chagas disease Press Release Drug Trials Snapshot |
| 30. | Vabomere | meropenem and vaborbactam | 8/29/2017 | To treat adults with complicated urinary tract infections Press Release Drug Trials Snapshot |
| 29. | Besponsa | inotuzumab ozogamicin | 8/17/2017 | To treat adults with relapsed or refractory acute lymphoblastic leukemia Press Release Drug Trials Snapshot |
| 28. | Mavyret | glecaprevir and pibrentasvir | 8/3/2017 | To treat adults with chronic hepatitis C virus Press Release Drug Trials Snapshot |
| 27. | Idhifa | enasidenib | 8/1/2017 | To treat relapsed or refractory acute myeloid leukemia Press Release Drug Trials Snapshot |
| 26. | Vosevi | sofosbuvir, velpatasvir and voxilaprevir | 7/18/2017 | To treat adults with chronic hepatitis C virus Press Release Drug Trials Snapshot |
| 25. | Nerlynx | neratinib maleate | 7/17/2017 | To reduce the risk of breast cancer returning Press Release Drug Trials Snapshot |
| 24. | Tremfya | guselkumab | 7/13/2017 | For the treatment of adult patients with moderate-to-severe plaque psoriasis Drug Trials Snapshot |
| 23. | Bevyxxa | betrixaban | 6/23/2017 | For the prophylaxis of venous thromboembolism (VTE) in adult patients hospitalized for an acute medical illness Drug Trials Snapshot |
| 22. | Baxdela | delafloxacin | 6/19/2017 | To treat patients with acute bacterial skin infections Drug Trials Snapshot |
| 21. | Kevzara | sarilumab | 5/22/2017 | To treat adult rheumatoid arthritis Drug Trials Snapshot |
| 20. | Radicava | edaravone | 5/5/2017 | To treat patients with amyotrophic lateral sclerosis (ALS) Press Release Drug Trials Snapshot |
| 19. | Imfinzi | durvalumab | 5/1/2017 | To treat patients with locally advanced or metastatic urothelial carcinoma Web Post Drug Trials Snapshot |
| 18. | Tymlos | abaloparatide | 4/28/2017 | To treat osteoporosis in postmenopausal women at high risk of fracture or those who have failed other therapies Drug Trials Snapshot |
| 17. | Rydapt | midostaurin | 4/28/2017 | To treat acute myeloid leukemia Press Release Chemistry Review(s) (PDF) Drug Trials Snapshot |
| 16. | Alunbrig | brigatinib | 4/28/2017 | To treat patients with anaplastic lymphoma kinase (ALK)-positive metastatic non-small cell lung cancer (NSCLC) who have progressed on or are intolerant to crizotinib Drug Trials Snapshot |
| 15. | Brineura | cerliponase alfa | 4/27/2017 | To treat a specific form of Batten disease Press Release Drug Trials Snapshot |
| 14. | Ingrezza | valbenazine | 4/11/2017 | To treat adults with tardive dyskinesia Press Release Chemistry Review(s) (PDF)Drug Trials Snapshot |
| 13. | Austedo | deutetrabenazine | 4/3/2017 | For the treatment of chorea associated with Huntington’s disease Drug Trials Snapshot, Chemistry Review(s) (PDF) |
| 12. | Ocrevus | ocrelizumab | 3/28/2017 | To treat patients with relapsing and primary progressive forms of multiple sclerosis Press Release Drug Trials Snapshot |
| 11. | Dupixent | dupilumab | 3/28/2017 | To treat adults with moderate-to-severe eczema (atopic dermatitis) Press Release Drug Trials Snapshot |
| 10. | Zejula | niraparib | 3/27/2017 | For the maintenance treatment for recurrent epithelial ovarian, fallopian tube or primary peritoneal cancers Press Release Drug Trials Snapshot |
| 9. | Symproic | naldemedine | 3/23/2017 |
For the treatment of opioid-induced constipation |
| 8. | Bavencio | avelumab | 3/23/2017 | To treat metastatic Merkel cell carcinoma Press Release Drug Trials Snapshot |
| 7. | Xadago | safinamide | 3/21/2017 | To treat Parkinson’s disease Press Release Drug Trials SnapshotChemistry Review(s) (PDF) |
| 6. | Kisqali | ribociclib | 3/13/2017 | To treat postmenopausal women with a type of advanced breast cancer Drug Trials Snapshot |
| 5. | Xermelo | telotristat ethyl | 2/28/2017 | To treat carcinoid syndrome diarrhea Press Release Drug Trials Snapshot |
| 4. | Siliq | brodalumab | 2/15/2017 | To treat adults with moderate-to-severe plaque psoriasis Press Release Drug Trials Snapshot |
| 3. | Emflaza | deflazacort | 2/9/2017 | To treat patients age 5 years and older with Duchenne muscular dystrophy (DMD) Press Release Drug Trials Snapshot |
| 2. | Parsabiv | etelcalcetide | 2/7/2017 | To treat secondary hyperparathyroidism in adult patients with chronic kidney disease undergoing dialysis Drug Trials Snapshot |
| 1. | Trulance | plecanatide | 1/19/2017 | To treat Chronic Idiopathic Constipation (CIC) in adult patients. Press Release Drug Trials Snapshot |
* This information is currently accurate. In rare instances, it may be necessary for FDA to change a drug’s new molecular entity (NME) designation or the status of its application as a novel new biologics license application (BLA). For instance, new information may become available which could lead to a reconsideration of the original designation or status. If changes must be made to a drug’s designation or the status of an application as a novel BLA, the Agency intends to communicate the nature of, and the reason for, any revisions as appropriate.
1 Abaloparatide
RADIUS

FDA 4/28/2017
To treat osteoporosis in postmenopausal women at high risk of fracture or those who have failed other therapies
Drug Trials Snapshot


2 Abemaciclib
ELI LILLY
| Verzenio | abemaciclib | FDA 9/28/2017 | To treat certain advanced or metastatic breast cancers Press Release Drug Trials Snapshot |
LINK https://newdrugapprovals.org/2015/10/19/abemaciclib-bemaciclib/
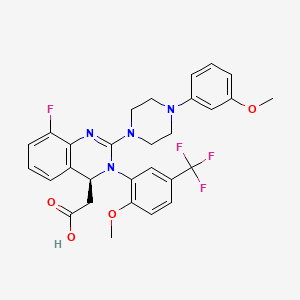
3 Acalabrutinib
| Calquence | FDA APPROVED
10/31/2017 |
To treat adults with mantle cell lymphoma Press Release Drug Trials Snapshot |

-Facebook.png)
4 Angiotensin II
LA JOLLA
| Giapreza | angiotensin II | 12/21/2017 | To increase blood pressure in adults with septic or other distributive shock Press Release Drug Trials Snapshot |


5 AVELUMAB
MERCK

| Bavencio | FDA 3/23/2017 | To treat metastatic Merkel cell carcinoma Press Release Drug Trials Snapshot |
6 BENRALIZUMAB
ASTRA ZENECA
Fasenra benralizumab
FDA 11/14/2017
For add-on maintenance treatment of patients with severe asthma aged 12 years and older, and with an eosinophilic phenotype
Drug Trials Snapshot


7 Benznidazole
CHEMO RESEARCH



| benznidazole | FDA
8/29/2017 |
To treat children ages 2 to 12 years old with Chagas disease Press Release Drug Trials Snapshot |
8 BETRIXABAN
PORTOLA PHARMA

| Bevyxxa | FDA
6/23/2017 |
For the prophylaxis of venous thromboembolism (VTE) in adult patients hospitalized for an acute medical illness Drug Trials Snapshot
|


9 BRIGATINIB
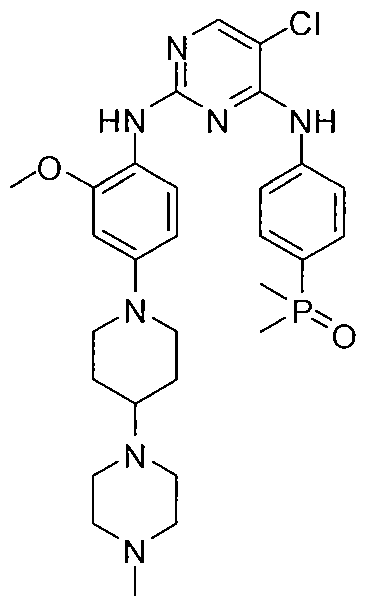
TAKEDA


| Alunbrig | FDA
4/28/2017 |
To treat patients with anaplastic lymphoma kinase (ALK)-positive metastatic non-small cell lung cancer (NSCLC) who have progressed on or are intolerant to crizotinib Drug Trials Snapshot |
10 BRODALUMAB
VALEANT PHARMA
| Siliq | FDA
2/15/2017 |
To treat adults with moderate-to-severe plaque psoriasis Press Release Drug Trials Snapshot |

LINK ,,,,https://newdrugapprovals.org/2017/02/16/fda-approves-new-psoriasis-drug-siliq-brodalumab/
11 CERLIPONASE ALFA

| Brineura | FDA 4/27/2017 | To treat a specific form of Batten disease Press Release Drug Trials Snapshot |
12 Copanlisib
| Aliqopa | FDA APPROVED
9/14/2017 |
To treat adults with relapsed follicular lymphoma Press Release Drug Trials Snapshot |


LINK…..https://newdrugapprovals.org/2017/11/20/copanlisib/
13 DEFLAZACORT
MARATHON PHARMA

| Emflaza | FDA 2/9/2017 | To treat patients age 5 years and older with Duchenne muscular dystrophy (DMD) Press Release Drug Trials Snapshot |

LINK……https://newdrugapprovals.org/2017/02/17/deflazacort/
14 DELAFLOXACIN
| Baxdela | FDA APPROVED
6/19/2017 |
To treat patients with acute bacterial skin infections |


LINK……..https://newdrugapprovals.org/2018/01/25/delafloxacin/
15 Deutetrabenazine
TEVA
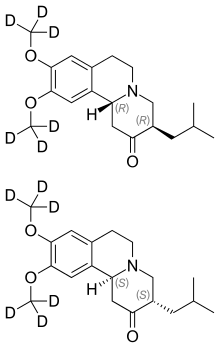


LINK……………https://newdrugapprovals.org/2015/08/15/sd-809-deutetrabenazine-nda-submitted-by-teva/
| Austedo | FDA 4/3/2017 | For the treatment of chorea associated with Huntington’s disease Drug Trials Snapshot Chemistry Review(s) (PDF) |
16 DUPILUMAB
SANOFI/REGENERON


| Dupixent | FDA | 3/28/2017 | To treat adults with moderate-to-severe eczema (atopic dermatitis) Press Release Drug Trials Snapshot |
LINK…….https://newdrugapprovals.org/2017/03/29/fda-approves-new-eczema-drug-dupixent-dupilumab/
17 DURVALUMAB
ASTRA ZENECA
durvalumab FDA 5/1/2017To treat patients with locally advanced or metastatic urothelial carcinoma
Web Post
Drug Trials Snapshot
18 EDAVARONE

MITSUBISHI TANABE
| Radicava | FDA 5/5/2017 | To treat patients with amyotrophic lateral sclerosis (ALS) Press Release Drug Trials Snapshot |


19 EMICIZUMAB
ROCHE

| Hemlibra | emicizumab | FDA 11/16/2017 | To prevent or reduce the frequency of bleeding episodes in adult and pediatric patients with hemophilia A who have developed antibodies called Factor VIII (FVIII) inhibitors. Press Release Drug Trials Snapshot |

20 Enasidenib


| Idhifa | FDA
8/1/2017 |
To treat relapsed or refractory acute myeloid leukemia Press Release Drug Trials Snapshot |
21 Ertugliflozin
MERCK
| Steglatro | ertugliflozin | FDA
12/19/2017 |
To improve glycemic control in adults with type 2 diabetes mellitus Drug Trials Snapshot |
LINK https://newdrugapprovals.org/2014/02/10/ertugliflozin/

22 ETELCALCETIDE
Amgen
| Parsabiv | FDA APPROVED
2/7/2017 |
To treat secondary hyperparathyroidism in adult patients with chronic kidney disease undergoing dialysis Drug Trials SnapshotSYNTHESIS LINK……..https://cen.acs.org/articles/96/i4/the-year-in-new-drugs-2018.html |
SYNTHESIS LINK……..https://cen.acs.org/articles/96/i4/the-year-in-new-drugs-2018.html
23 GLECAPREVIR
ABBVIE

| Mavyret | glecaprevir and pibrentasvir | FDA 8/3/2017 | To treat adults with chronic hepatitis C virus Press Release Drug Trials Snapshot |
LINK https://newdrugapprovals.org/2016/10/05/glecaprevir-abt-493/
24 GUSELKUMAB
JOHNSON AND JOHNSON
guselkumab
FDA 7/13/2017
For the treatment of adult patients with moderate-to-severe plaque psoriasis
Drug Trials Snapshot


25 Inotuzumab ozogamicin
PFIZER



| Besponsa | FDA
8/17/2017 |
To treat adults with relapsed or refractory acute lymphoblastic leukemia Press Release Drug Trials Snapshot |
26 LATANOPROSTENE
VALEANT
latanoprostene bunod ophthalmic solution
FDA 11/2/2017
To treat intraocular pressure in patients with open-angle glaucoma or ocular hypertension.
Drug Trials Snapshot
27 LETERMOVIR
MERCK
| Prevymis | FDA 11/8/2017 | To prevent infection after bone marrow transplant Drug Trials Snapshot |
LINK https://newdrugapprovals.org/2016/05/16/letermovir-aic-246/
28 Macimorelin acetate
AETERNA ZENTARIS
| Macrilen | macimorelin acetate | FDA
12/20/2017 |
For the diagnosis of adult growth hormone deficiency Drug Trials Snapshot |
29 MEROPENEM

30 MIDOSTAURIN
NOVARTIS
- Chemistry Review(s) (PDF)

| Rydapt | FDA
4/28/2017 |
To treat acute myeloid leukemia Press Release Drug Trials Snapshot |
31 Naldemedine
FDA 3/23/2017, Symproic, For the treatment of opioid-induced constipation


32 NERATINIB MALEATE
PUMA BIOTECH



| Nerlynx | FDA | 7/17/2017 | To reduce the risk of breast cancer returning Press Release Drug Trials Snapshot |
33 NETARSUDIL
| Rhopressa | FDA APPROVED
12/18/2017 |
To treat glaucoma or ocular hypertension |


LINK……https://newdrugapprovals.org/2018/01/29/netarsudil/
34 NIRAPARIB
TESARO
| Zejula | FDA | 3/27/2017 | For the maintenance treatment for recurrent epithelial ovarian, fallopian tube or primary peritoneal cancers Press Release Drug Trials Snapshot |



LINK…https://newdrugapprovals.org/2016/12/22/niraparib-mk-4827/
35 OCRELIZUMAB
ROCHE
| Ocrevus | FDA | 3/28/2017 | To treat patients with relapsing and primary progressive forms of multiple sclerosis Press Release Drug Trials Snapshot |


36 OZENOXACIN
MEDIMETRIX

| Xepi | ozenoxacin | FDA
12/11/2017 |
To treat impetigo Drug Trials Snapshot |
37 Pibrentasvir
ABBVIE

| Mavyret | glecaprevir and pibrentasvir | FDA 8/3/2017 | To treat adults with chronic hepatitis C virus Press Release Drug Trials Snapshot |
LINK https://newdrugapprovals.org/2016/06/08/abt-530-pibrentasvir/
38 PLECANATIDE
Plecanatide 普卡那肽 ليكاناتيد плеканатид
SYNERGY PHARMA


| Trulance | FDA APPROVED
1/19/2017 |
To treat Chronic Idiopathic Constipation (CIC) in adult patients. Press Release Drug Trials Snapshot |
39 RIBOCICLIB
NOVARTIS
Structure..link for correct structure
| Kisqali | FDA 3/13/2017 | To treat postmenopausal women with a type of advanced breast cancer Drug Trials Snapshot |

LINK https://newdrugapprovals.org/2015/10/19/ribociclib/
40 SARILUMAB
SANOFI /REGENERON

| Kevzara | sarilumab | FDA 5/22/2017 | To treat adult rheumatoid arthritis Drug Trials Snapshot |
LINK https://newdrugapprovals.org/2013/11/25/late-stage-success-for-sanofiregeneron-ra-drug-sarilumab/


41 SECNIDAZOLE
SYMBIOMIX
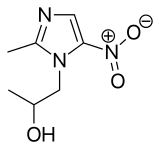
| Solosec | FDA | 9/15/2017 | To treat bacterial vaginosis Drug Trials Snapshot |

42 SAFINAMIDE
NEWRON PHARMA



- Chemistry Review(s) (PDF) for correct structure
| Xadago | FDA 3/21/2017 | To treat Parkinson’s disease Press Release Drug Trials Snapshot |
43 Semaglutide
NOVO NORDISK
| Ozempic | semaglutide | FDA
12/5/2017 |
To improve glycemic control in adults with type 2 diabetes mellitus Drug Trials Snapshot |
LINK https://newdrugapprovals.org/2013/07/22/a-survey-of-promising-late-stage-diabetes-drugs/

44 SOFOSBUVIR
45 TELOTRISTAT ETHYL
LEXICON
| Xermelo | FDA
2/28/2017 |
To treat carcinoid syndrome diarrhea Press Release Drug Trials Snapshot |


46 VABORBACTAM
THE MEDICINES CO

| Vabomere | meropenem and vaborbactam | FDA
8/29/2017 |
To treat adults with complicated urinary tract infections Press Release Drug Trials Snapshot |

47 VALBENAZINE
NEUROCRINE
- Chemistry Review(s) (PDF)


| Ingrezza | FDA
4/11/2017 |
To treat adults with tardive dyskinesia Press Release Drug Trials Snapshot |
48 Vestronidase alfa-vjbk
ULTRAGENYX
| Mepsevii | vestronidase alfa-vjbk | FDA 11/15/2017 | To treat pediatric and adult patients with an inherited metabolic condition called mucopolysaccharidosis type VII (MPS VII), also known as Sly syndrome. Press Release Drug Trials Snapshot |


49 VELPATASVIR
50 VOXILAPREVIR
GILEAD


| Vosevi | sofosbuvir, velpatasvir and voxilaprevir | FDA 7/18/2017 | To treat adults with chronic hepatitis C virus Press Release Drug Trials Snapshot |
SECTION B; EMA approvals
European Medicines Agency’s – Human medicines: Highlights of 2017
Advances in medicines authorizations are essential for public health as they have the potential to improve treatment of diseases. In 2017, EMA recommended 92 medicines for marketing authorization. Of these, 35 had a new active substance, which has never been authorized in the European Union (EU) before. Many of these medicines represent a significant improvement in their therapeutic areas; they include medicines for children, for rare diseases and advanced therapies42. Amongst the 35 new active substances (NAS) that EMA recommended, 11 were new drugs and biologics to treat cancer, 05 to treat neurological disorders, 04 for infectious diseases, 04 for immunology/rheumatology, 03 for endocrinology, 02 each for Uro-nephrology, haematology, and dermatology, 01 for Pneumonology, and 01 for hepatology/gastroenterology class of drugs.
SECTION B; EMA Approvals
Combined drugs USFDA+EMA list are listed below. trying to simplify search
1 Abaloparatide USFDA
2 Abemaciclib USFDA
3 ACALABRUTINIB USFDA
3A ALOFISEL EMA
4 ANGIOTENSIN II USFDA
4A Atezolizumab EMA
5 AVELUMAB USFDA+EMA
6 BENRALIZUMAB USFDA+EMA
7 BENZNIDAZOLE USFDA
8 BETRIXABAN USFDA
9 BRIGATINIB USFDA
10 BRODALUMAB USFDA+EMA
10A BUROSUMAB EMA
10B CARIPRAZINE HYDROCHLORIDE EMA
11 CERLIPONASE ALPA USFDA+EMA
12 COPANLISIB USFDA
13 DEFLAZACORT USFDA
14 Delafloxacin USFDA
15 Deutetrabenazine USFDA
16DUPILUMAB USFDA+EMA
17 DURVALUMAB USFDA
18 EDAVARONE USFDA
19 EMICIZUMAB USFDA
20 Enasidenib USFDA
21 ERTUGLIFLOZIN USFDA
22 ETELCALCETIDE USFDA
22A FLUCICLOVINE EMA
23 GLECAPREVIR USFDA+EMA
24 GUSELKUMAB USFDA+EMA
25 INOTUZUMAB OZOGAMICIN USFDA+EMA
26 LATANOPROSTENE USFDA
27 LETERMOVIR USFDA+EMA
27A Utetium lu 177 dotatate EMA
28 MACIMORELIN ACETATE USFDA
29 MEROPENEM USFDA
30 MIDOSTAURIN USFDA+EMA
31 NALDEMEDINE USFDA
32 NERATINIB USFDA
33 NETARSUDIL USFDA
34A NONACOG EMA
34B NUCINERSEN EMA
35 Ocrelizumab USFDA+EMA
35A OXERVATE EMA
36 OZENOXACIN USFDA
36A PATIROMER EMA
36B PADELIPORFIN EMA
37 PIBRENTASVIR USFDA+EMA
38 PLECANATIDE USFDA
39A ROLAPITANT EMA
39B RURLOCTOCOG EMA
40 SARILUMAB USFDA+EMA
41 SECNIDAZOLE USFDA
42 SAFINAMIDE USFDA
43 SEMAGLUTIDE USFDA+EMA
43A SODIUM ZIRCONIUM CYCLOCYLICATE EMA
44 SOFOSBUVIR USFDA+EMA
44A SPHEROX EMA
45 TELOTRISTAT ETHYL USFDA+EMA
45A TIVOZANIB EMA
45B TOFACITINIB EMA
45C TRUMENBA EMA
46 VABORBACTAM USFDA
47 VALBENAZINE USFDA
48 VESTRONIDASE ALFA-VJBK USFDA
49 VELPATASVIR USFDA+EMA
50 VOXILAPREVIR USFDA+EMA
Drugs EMA list missed out in usfda list
3A ALOFISEL

link………https://newdrugapprovals.org/2018/03/02/alofisel-darvadstrocel-cx-601/
4A Atezolizumab
10A BUROSUMAB
10B CARIPRAZINE HYDROCHLORIDE
22A FLUCICLOVINE

SEE EMA
| Axumin : EPAR – Summary for the public | EN = English | 06/07/2017 |
27A lutetium lu 177 dotatate
34A NONACOG
34B NUCINERSEN
35A OXERVATE
36A PATIROMER
36B PADELIPORFIN

| NAME | Tookad |
|---|---|
| AGENCY PRODUCT NUMBER | EMEA/H/C/004182 |
| ACTIVE SUBSTANCE | padeliporfin di-potassium |
| INTERNATIONAL NON-PROPRIETARY NAME(INN) OR COMMON NAME | padeliporfin |
| THERAPEUTIC AREA | Prostatic Neoplasms |
| ANATOMICAL THERAPEUTIC CHEMICAL (ATC) CODE | L01XD07 |
| ADDITIONAL MONITORING | This medicine is under additional monitoring. This means that it is being monitored even more intensively than other medicines. For more information, see medicines under additional monitoring. |
| MARKETING-AUTHORISATION HOLDER | STEBA Biotech S.A |
|---|---|
| REVISION | 0 |
| DATE OF ISSUE OF MARKETING AUTHORISATION VALID THROUGHOUT THE EUROPEAN UNION | 10/11/2017 |
Contact address:
STEBA Biotech S.A
7 place du theatre
L-2613 Luxembourg
Luxembourg
39A ROLAPITANT
39B RURLOCTOCOG
43A SODIUM ZIRCONIUM
44A SPHEROX
45A TIVOZANIB

Pharmacotherapeutic group
Antineoplastic agents
Therapeutic indication
Fotivda is indicated for the first line treatment of adult patients with advanced renal cell carcinoma (RCC) and for adult patients who are VEGFR and mTOR pathway inhibitor-naïve following disease progression after one prior treatment with cytokine therapy for advanced RCC.
Treatment of advanced renal cell carcinoma
Fotivda : EPAR -Product Information

Tivozanib is synthesized in three main steps using well defined starting materials with acceptable
specifications.
Adequate in-process controls are applied during the synthesis. The specifications and control methods for
intermediate products, starting materials and reagents have been presented. The critical process
parameters are duly justified, methodology is presented and control is adequate.
The characterisation of the active substance and its impurities are in accordance with the EU guideline on
chemistry of new active substances. Potential and actual impurities were well discussed with regards to
their origin and characterised.
The active substance is packaged in a low-density polyethylene (LDPE) bag which complies with the EC
directive 2002/72/EC and EC 10/2011 as amended.
Product details
| Name | Fotivda |
|---|---|
| Agency product number | EMEA/H/C/004131 |
| Active substance | tivozanib |
| International non-proprietary name(INN) or common name | tivozanib hydrochloride monohydrate |
| Therapeutic area | Carcinoma, Renal Cell |
| Anatomical therapeutic chemical (ATC) code | L01XE |
Publication details
| Marketing-authorisation holder | EUSA Pharma (UK) Limited |
|---|---|
| Revision | 0 |
| Date of issue of marketing authorisation valid throughout the European Union | 24/08/2017 |
Contact address:
EUSA Pharma (UK) Limited
Breakspear Park, Breakspear Way
Hemel Hempstead, HP2 4TZ
United Kingdom
45B TOFACITINIB
45C TRUMENBA
KEEP WATCHING UNDER CONSTRUCTION AND WILL BE PASTED SOON………………………………………..
KEEP WATCHING UNDER CONSTRUCTION AND WILL BE PASTED SOON………………………………………..
KEEP WATCHING UNDER CONSTRUCTION AND WILL BE PASTED SOON………………………………………..
KEEP WATCHING UNDER CONSTRUCTION AND WILL BE PASTED SOON………………………………………..
REFERENCES
2 http://www.ema.europa.eu/docs/en_GB/document_library/Report/2018/01/WC500242079.pdf
“NEW DRUG APPROVALS” CATERS TO EDUCATION GLOBALLY, No commercial exploits are done or advertisements added by me. This is a compilation for educational purposes only. P.S. : The views expressed are my personal and in no-way suggest the views of the professional body or the company that I represent
![]() amcrasto@gmail.com
amcrasto@gmail.com
I , Dr A.M.Crasto is writing this blog to share the knowledge/views, after reading Scientific Journals/Articles/News Articles/Wikipedia. My views/comments are based on the results /conclusions by the authors(researchers). I do mention either the link or reference of the article(s) in my blog and hope those interested can read for details. I am briefly summarising the remarks or conclusions of the authors (researchers). If one believe that their intellectual property right /copyright is infringed by any content on this blog, please contact or leave message at below email address amcrasto@gmail.com. It will be removed ASAP
////////EMA APPROVALS, USFDA Approvals, ACALABRUTINIB, AVELUMAB, BETRIXABAN, BRODALUMAB, COPANLISIB, DEFLAZACORT, Delafloxacin, Deutetrabenazine, DUPILUMAB, ETELCALCETIDE, Naldemedine, NETARSUDIL, NIRAPARIB, Ocrelizumab, PLECANATIDE, RIBOCICLIB, SAFINAMIDE, TELOTRISTAT ETHYL, VALBENAZINE, CERLIPONASE, BRIGATINIB, MIDOSTAURIN, Abaloparatide, BENZNIDAZOLE, NERATINIB, inotuzumab ozogamicin, Enasidenib, LETERMOVIR, GLECAPREVIR, PIBRENTASVIR, VOXILAPREVIR, SOFOSBUVIR, EDAVARONE, abemaciclib, ANGIOTENSIN II, VESTRONIDASE, macimorelin acetate, ERTUGLIFLOZIN, SEMAGLUTIDE, EMICIZUMAB, eu 2017, fda 2017, BENRALIZUMAB, DURVALUMAB, GUSELKUMAB, LATANOPROSTENE, OZENOXACIN, SARILUMAB, SECNIDAZOLE, BENRALIZUMAB, TIVOZANIB, SARILUMAB, FLUCICLOVINE,