DRUG REGULATORY AFFAIRS INTERNATIONAL
ICH Q3D Implementation Working Group (IWG)—Training Modules
View original post 1,045 more words
Filed under: Uncategorized
DRUG REGULATORY AFFAIRS INTERNATIONAL
View original post 1,045 more words
DRUG REGULATORY AFFAIRS INTERNATIONAL

The US Food and Drug Administration’s (FDA) Office of Pharmaceutical Quality (OPQ) released a new document outlining how supplements can be grouped together and submitted concurrently for the same chemistry, manufacturing and controls (CMC) changes. Find out more about Policy and Procedures regarding the Review of Grouped Product Quality Supplements.
On April 19, 2016 the US Food and Drug Administration’s (FDA) Office of Pharmaceutical Quality (OPQ) released a new document outlining how supplements can be grouped together and submitted concurrently for the same chemistry, manufacturing and controls (CMC) changes to multiple approved new drug applications (NDAs), abbreviated new drug applications (ANDAs) and biological license applications (BLAs) submitted by the same applicant.
The agency says the goal of its new policy is to make the process more efficient and consistent when reviewing grouped supplements.The term “grouped supplements” is used to describe two or more supplements reviewed and processed using…
View original post 343 more words
DRUG REGULATORY AFFAIRS INTERNATIONAL

The European Medicines Agency, EMA, recently published questions and answers on what data is required for sterilisation processes of primary packaging materials subsequently used in an aseptic manufacturing process. Read more about “What data is required for sterilisation processes of primary packaging materials subsequently used in an aseptic manufacturing process?“.
The European Medicines Agency, EMA, recently published questions and answers on quality of packaging materials (H+V April 2016):
“3. What data is required for sterilisation processes of primary packaging materials subsequently used in an aseptic manufacturing process?
Terminal sterilisation of the primary packaging, used subsequently during aseptic processing of the finished product, is a critical process and the sterility of the primary container is a critical quality attribute to ensure the sterility of the finished product. Both need to be assured for compliance with relevant Pharmacopoeial requirements for the finished product and product approval.
The site where sterilisation…
View original post 556 more words
DRUG REGULATORY AFFAIRS INTERNATIONAL


View original post 1,776 more words

2-[2-Methyl-1-(tetrahydro-2H-pyran-4-yl)-1H-benzimidazol-5-yl]-1,3-benzoxazole Hemifumarate
Sumitomo Dainippon Pharma Company,
CAS FREE FORM 1256966-65-0
Benzoxazole, 2-[2-methyl-1-(tetrahydro-2H-pyran-4-yl)-1H-benzimidazol-5-yl]-
1H NMR (400 MHz, DMSO-d6)
13C NMR (100 MHz, DMSO-d6)

A short and practical synthetic route of a PDE4 inhibitor (1) was established by using Pd–Cu-catalyzed C–H/C–Br coupling of benzoxazole with a heteroaryl bromide. The combination of Pd(OAc)2-Cu(OTf)2-PPh3 was found to be effective for this key step. Furthermore, telescoping methods were adopted to improve the yield and manufacturing time, and a two-step synthesis of1 was accomplished in 71% overall yield.
///////////PDE4 inhibitor , Sumitomo Dainippon Pharma Company
Cc1nc3cc(ccc3n1C2CCOCC2)c4nc5ccccc5o4


Higenamine Hydrochloride
NDA Filed in china
A β-adrenoceptor partial agonist potentially for the treatment of coronary heart disease.


CAS No.11041-94-4 (Higenamine hydrochloride)

CAS 5843-65-2(free)
Higenamine (norcoclaurine) is a chemical compound found in a variety of plants including Nandina domestica (fruit), Aconitum carmichaelii (root), Asarum heterotropioides, Galium divaricatum (stem and vine), Annona squamosa, and Nelumbo nucifera (lotus seeds).
Higenamine, also known as norcoclaurine HCl, is legal to use within food supplements in the UK, EU, the USA and Canada. but banned use in The NCAA. Its main is within food supplements developed for weight management, also known as ‘fat burners’. However, products containing (or claiming to contain) pharmacological relevant quantities still require registration as a medicine. The regulatory boundaries for higenamine are unclear as modern formulations have not been clinically evaluated. Traditional formulations with higenamine have been used for thousands of years within Chinese medicine and come from a variety of sources including fruit and orchids. There are no studies comparing the safety of modern formulations (based on synthetic higenamine) with traditional formulations. Nevertheless, it will not be added to the EU ‘novel foods’ catalogue, which details all food supplements that require a safety assessment certificate before use.[1]

Since higenamine is present in plants which have a history of use in traditional medicine, the pharmacology of this compound has attracted scientific interest. A variety of effects have been observed in in vitro studies and in animal models, but its effects in humans are unknown.
The results of a 2009 study exposed the compound as a β2 adrenergic receptor agonist.[2]
In animal models, higenamine has been demonstrated to be a β2 adrenoreceptor agonist.[2][3][4][5][6] Adrenergic receptors, or adrenoceptors, belong to the class of G protein–coupled receptors, and are the most prominent receptors in the adipose membrane, besides also being expressed in skeletal muscle tissue. These adipose membrane receptors are classified as either α or β adrenoceptors. Although these adrenoceptors share the same messenger, cyclic adenosine monophosphate (cAMP), the specific transduction pathway depends on the receptor type (α or β). Higenamine partly exerts its actions by the activation of an enzyme,adenylate cyclase, responsible for boosting the cellular concentrations of the adrenergic second messenger, cAMP.[7]
In a rodent model, it was found that higenamine produced cardiotonic, vascular relaxation, and bronchodilator effects.[8][9] In particular, higenamine, via a beta-adrenoceptor mechanism, induced relaxation in rat corpus cavernosum, leading to improved vasodilation and erectile function.
Related to improved vasodilatory signals, higenamine has been shown in animal models to possess antiplatelet and antithrombotic activity via a cAMP-dependent pathway, suggesting higenamine may contribute to enhanced vasodilation and arterial integrity.[2][7][9][10]
Regarding toxicity, researchers have suggested that the levels of higenamine reported in food consumption (estimated 47.5 mg in a 9-ounce serving of Lotus) would be comparable to the amount used in food supplements.[citation needed] Higenamine is a beta-adrenergic agonist which has effects on the function of trachea and heart muscles.[11][12]During a study of acute toxicity, mice were orally administered the compound at a dose of 2 g per kg of bodyweight. No mice died during the study.[13] higenamine is one of the main chemicals in a plant called aconite. Aconite has been shown to cause serious heart-related side effects including arrhythmias and even death. in some sources of HIGENAMINE from certain plants that have Aconite
PAPER
Chemical & Pharmaceutical Bulletin (1978), 26(7), 2284-5
https://www.jstage.jst.go.jp/article/cpb1958/26/7/26_7_2284/_pdf
PATENT
CN 103554022
http://google.com/patents/CN103554022B?cl=en
Example 1:
[0024] to the S-necked flask 200mL of anhydrous ammonia clever four furans, lOg instrument crumbs, olive mix was added 0. 5g ship, continue to embrace the mix was added 10 minutes after which 2 drops of 1,2-B burning desert, Continue mixing until the reaction mixture embrace color disappeared, the reaction was cooled to square ° C, and slowly mixed solution thereto 31. 6g4- methoxy Desert Festival and 50mL tetraammine clever furans dropped, about 60min addition was complete, the reaction fluid continues to cool to -65 ° C, to which was slowly dropping 20 percent, 7-dimethoxy-3,4-diamine different wow beep and a mixed solution of ammonia lOOmL four clever furans, the addition was complete continue to maintain – 65 ° C for 2 hours after the embrace slowly warmed 0 ° C, maintaining the internal temperature of 100 ° C 〇 blood slowly added to the reaction mixture, the addition was completed adding 200 blood continues to embrace mixed with ethyl acetate after 0.5 hours, allowed to stand liquid separation, organic phase was separated, dried over anhydrous sulfate steel, concentrated to afford 6, 7-dimethoxy -l- (4- methoxy section yl) -1,2, 3, 4-isopropyl tetraammine wow toot 24. 9g, a yield of 76.1%.
[00 Qiao] to the reaction flask prepared above 6, 7-dimethoxy -l- (4- methoxybenzyl) -1,2, 3, 4 tetraammine different wow beep 24. 9g , 47% aqueous ammonia desert 200 blood acid heated to 130 ° C reflux of cooled to room temperature, precipitation of large amount of solid, filtered higenamine ammonia salt desert, the solid was added 1. of water and continue to add 50 Blood mixed with ammonia football ground, filtered higenamine to higenamine was added lL4mol / L aqueous hydrochloric acid, 80 ° C heat to embrace mixed, cooled to 25 ° C filtration and drying to obtain a final product hydrochloric acid higenamine 11. 7g, a yield of 73.3%.
banned in ncaa https://www.ncaa.org/sites/default/files/2015-16%20NCAA%20Banned%20Drugs.pdf
| CN1539823A * | Oct 27, 2003 | Oct 27, 2004 | 中国医学科学院药物研究所 | Method for preparing new demethyl conclaurine and medinal salt |
| CN1764647A * | Mar 23, 2004 | Apr 26, 2006 | 埃科特莱茵药品有限公司 | Tetrahydroisoquinolyl acetamide derivatives for use as orexin receptor antagonists |
| CN103351338A * | Jun 17, 2013 | Oct 16, 2013 | 张家港威胜生物医药有限公司 | Simple preparation process of higenamine hydrochloride |
| US20060030586 * | Sep 27, 2004 | Feb 9, 2006 | Education Center Of Traditional Chinese Medicine Co. | Method and health food for preventing and/or alleviating psychiatric disorder, and/or for effectuating sedation |
| WO2011038169A2 * | Sep 24, 2010 | Mar 31, 2011 | Mallinckrodt Inc. | One-pot preparation of hexahydroisoquinolines from amides |
 |
|
| Names | |
|---|---|
| IUPAC name
1-[(4-Hydroxyphenyl)methyl]-1,2,3,4-tetrahydroisoquinoline-6,7-diol
|
|
| Other names
norcoclaurine, demethylcoclaurine
|
|
| Identifiers | |
5843-65-2  106032-53-5 (R)  22672-77-1 (S)  |
|
| ChEBI | CHEBI:18418  |
| ChEMBL | ChEMBL19344  |
| ChemSpider | 102800  |
| Jmol 3D model | Interactive image |
| KEGG | C06346  |
| MeSH | higenamine |
| PubChem | 114840 |
| Properties | |
| C16H17NO3 | |
| Molar mass | 271.32 g·mol−1 |
/////
DRUG REGULATORY AFFAIRS INTERNATIONAL

Dr Friedrich Haefele, Vice President Fill & Finish Biopharma at Boehringer Ingelheim
Dr Friedrich Haefele, Vice President Fill & Finish Biopharma at Boehringer Ingelheim talked in his keynote speech at the Pharma Congress 2016 about the revision of Annex 1 of the EU GMP Guide. Read here what the pharmaceutical industry expects form the new Annex 1.
Europe’s biggest Pharma Congress of its kind took place in Düsseldorf on 12 and 13 April. With more than 1000 participants, 90 exhibitors and 10 GMP conferences this Congress 2016 has been the biggest since the first one 18 years ago. 50 lectures, almost exclusively case studies from pharmacuetical companies such as Pfizer, Novartis, Boehringer Ingelheim and many more were discussed. Special attention was paid to the keynotes at the beginning of each congress day.
 Dr Friedrich Haefele, Vice President Fill & Finish Biopharma at Boehringer Ingelheim talked in his keynote…
Dr Friedrich Haefele, Vice President Fill & Finish Biopharma at Boehringer Ingelheim talked in his keynote…
View original post 876 more words
DRUG REGULATORY AFFAIRS INTERNATIONAL

The World Health Organization (WHO) recently issued a guideline for commenting which describes the requirements for HVAC systems for the manufacture of non-sterile forms. As most guidelines on this topic address the requirements for sterile dosage forms, the previous version was gladly accepted by industry. Learn more about the revised guideline on HVAC systems.
The World Health Organization (WHO) recently issued a guideline for commenting which describes the requirements for HVAC systems used for the manufacture of non-sterile dosage forms. As most guidelines on this topic address the requirements for sterile forms, the previous version (TRS 961, Annex 1) from 2011 was gladly accepted by industry. Mentioned are non-sterile dosage forms as tablets, capsules, liquids or ointments, but also for the final steps in the manufacture of APIs. The WHO guideline means to provide guidance specifically for the areas design, installation, qualification and maintenance of ventilation systems. For the manufacture of…
View original post 192 more words
NOTE………CAS OF AMG 580 IS 1227067-71-1, WITHOUT 18F
AMG 580 [1-(4-(3-(4-(1H-benzo[d]imidazole-2-carbonyl)phenoxy)pyrazin-2-yl)piperidin-1-yl)-2-fluoropropan-1-one],
Phosphodiesterase 10A (PDE10A) inhibitors have therapeutic potential for the treatment of psychiatric and neurologic disorders, such as schizophrenia and Huntington’s disease. One of the key requirements for successful central nervous system drug development is to demonstrate target coverage of therapeutic candidates in brain for lead optimization in the drug discovery phase and for assisting dose selection in clinical development. Therefore, we identified AMG 580 [1-(4-(3-(4-(1H-benzo[d]imidazole-2-carbonyl)phenoxy)pyrazin-2-yl)piperidin-1-yl)-2-fluoropropan-1-one], a novel, selective small-molecule antagonist with subnanomolar affinity for rat, primate, and human PDE10A. We showed that AMG 580 is suitable as a tracer for lead optimization to determine target coverage by novel PDE10A inhibitors using triple-stage quadrupole liquid chromatography–tandem mass spectrometry technology. [3H]AMG 580 bound with high affinity in a specific and saturable manner to both striatal homogenates and brain slices from rats, baboons, and human in vitro. Moreover, [18F]AMG 580 demonstrated prominent uptake by positron emission tomography in rats, suggesting that radiolabeled AMG 580 may be suitable for further development as a noninvasive radiotracer for target coverage measurements in clinical studies. These results indicate that AMG 580 is a potential imaging biomarker for mapping PDE10A distribution and ensuring target coverage by therapeutic PDE10A inhibitors in clinical studies.
We report the discovery of PDE10A PET tracer AMG 580 developed to support proof of concept studies with PDE10A inhibitors in the clinic. To find a tracer with higher binding potential (BPND) in NHP than our previously reported tracer 1, we implemented a surface plasmon resonance assay to measure the binding off-rate to identify candidates with slower washout rate in vivo. Five candidates (2–6) from two structurally distinct scaffolds were identified that possessed both the in vitro characteristics that would favor central penetration and the structural features necessary for PET isotope radiolabeling. Two cinnolines (2, 3) and one keto-benzimidazole (5) exhibited PDE10A target specificity and brain uptake comparable to or better than 1 in the in vivo LC–MS/MS kinetics distribution study in SD rats. In NHP PET imaging study, [18F]-5 produced a significantly improved BPND of 3.1 and was nominated as PDE10A PET tracer clinical candidate for further studies.
PATENT FOR AMG 580
WO 2010057121
https://www.google.com/patents/WO2010057121A1?cl=en
PAPER
Nuclear Medicine and Biology (2015), 42(8), 654-663.
http://www.sciencedirect.com/science/article/pii/S0969805115000724
Phosphodiesterase 10A (PDE10A) is an intracellular enzyme responsible for the breakdown of cyclic nucleotides which are important second messengers for neurotransmission. Inhibition of PDE10A has been identified as a potential target for treatment of various neuropsychiatric disorders. To assist drug development, we have identified a selective PDE10A positron emission tomography (PET) tracer, AMG 580. We describe here the radiosynthesis of [18 F]AMG 580 and in vitro and in vivo characterization results.
AMG 580 has an in vitro KD of 71.9 pM. Autoradiography showed specific uptake in striatum. Mean activity of 121 ± 18 MBq was used in PET studies. In Rhesus, the baseline BPND for putamen and caudate was 3.38 and 2.34, respectively, via 2TC, and 3.16, 2.34 via Logan, and 2.92, and 2.01 via SRTM. A dose dependent decrease of BPNDwas observed by the pre-treatment with a PDE10A inhibitor. In baboons, 0.24 mg/kg dose of AMG 580 resulted in about 70% decrease of BPND. The in vivo KD of [18 F]AMG 580 was estimated to be around 0.44 nM in baboons.
[18 F]AMG 580 is a selective and potent PDE10A PET tracer with excellent specific striatal binding in non-human primates. It warrants further evaluation in humans.
REFERNCES
http://jpet.aspetjournals.org/content/352/2/327.full
///Phosphodiesterase, tracer, receptor occupancy, positron emission tomography, radiotracer, brain penetration, AMG 580, Phosphodiesterase 10A, PDE10A, PET Tracer, [18F]AMG 580
CAS 1196963-79-7
| [18F](2S,4S)-4-FPGln |
[18F](2S,4S)-4-(3-fluoropropyl)glutamine, 4 |
The early diagnosis of malignant tumors plays a very important role in the survival prognosis of cancer patients. In this non-invasive diagnosis, diagnostic imaging procedures are an important tool. In the last few years has mainly PET technology (P ositronen- E mission- Tomographie) proved to be particularly useful. The sensitivity and specificity of PET technology depends significantly on the used signal-emitting substance (tracer) and their distribution in the body from. In the search for suitable tracers one tries to take advantage of certain properties of tumors differ, the tumor tissue from healthy, surrounding tissue. The preferred commercially used isotope which finds application for PET, 18 F 18 F represents by its short half-life of less than 2 hours special requirements for the preparation of suitable tracer. Complex, long synthetic routes and purifications are with this isotope is not possible, because otherwise a significant portion of the radioactivity of the isotope has already decayed before the tracer can be used for diagnosis. It is therefore often not possible to established synthetic routes for non-radioactive fluorination to be applied to the synthesis of18 F-tracer. Furthermore, the high specific activity of 18 F (80 GBq / nmol) at very low substance amounts of [18 F] fluoride for the tracer synthesis, which in turn an extreme excess of precursor-related and the success of a non-radioactive fluorination based Radio synthetic strategy designed unpredictable
FDG ([18 F] F 2 luoro d esoxy lukose g) -PET is a widely accepted and popular tool in the diagnosis and other clinical tracking of tumor diseases. Malignant tumors compete with the host organism to glucose supply to the nutrient supply (Warburg O. About the metabolism of carcinoma cell Biochem;.. Kellof G. Progress and Promise of FDG PET Imaging for Cancer Patient Management and Oncologic Drug Development Clin Cancer Res 2005;.. 11 (8): 2785-2807) where tumor cells compared to surrounding cells of normal tissue usually an increased glucose metabolism. This is used when using fluorodeoxyglucose (FDG), a glucose derivative, which is amplified transported into the cells, but there included metabolically after phosphorylation as FDG-6-phosphate (“Warburg effect”). 18 F-labeled FDG is Therefore, an effective tracer for the detection of tumors in patients using PET technology. Imaging were looking for new PET tracers in recent years increasingly amino acids for 18 F PET used (eg (review): Eur J Nucl Med Mol Imaging 2002 May; 29 (5):.. 681-90). In this case, some of the 18 F-labeled amino acids for the measurement of the speed rate of protein synthesis, the most useful derivatives but for the direct measurement of the cellular uptake in the tumor. Known 18 F-labeled amino acids are, for example, from tyrosine, phenylalanine, proline, aspartic and unnatural amino acids derived (eg J. Nucl Med 1991; 32:.. 1338-1346, J Nucl Med 1996; 37: 320-325, J Nucl Med 2001; 42: 752-754 J Nucl Med and 1999, 40: 331-338).. Glutamic acid and glutamine than 18 F-labeled derivatives not known, whereas non-radioactive fluorinated glutamine and glutamic acid derivatives are well known; Thus, for Example those which at γ-position (for Ex (review):Amino Acids (2003) April; 24 (3):… 245-61).. or at β-position (e.g. ExTetrahedron. Lett. .; 30; 14; 1989, 1799-1802, J. Org Chem .; 54; 2; 1989, 498-500, Tetrahedron: Asymmetry, 12, 9; 2001; 1303-1312) havefluorine..
Of glutamic acid having the chemical functionalities protecting groups in β and γ position or a leaving group, has already been reported in the past. So was informed of glutamate as mesylate or bromide in γ-position whose acid and amine functions were provided with ester or Z-protecting groups (J. Chem Soc Perkin Trans. 1;.. 1986, 1323-1328) or, for example, of γ-chloro-glutamic acid without protecting groups(Synthesis, (1973); 44-46). About similar derivatives, but where the leaving group is positioned in β-position has also been reported on several occasions. Z Ex. Chem. Pharm. Bull .; 17; 5; (1969); 879-885,J.Gen.Chem.USSR (Engl.Transl.); 38; (1968); 1645-1648, Tetrahedron Lett .; 27; 19; (1986); 2143-2144, Chem. Pharm. Bull .; EN; 17; 5; 1969;873-878, patent FR 1461184 , Patent JP 13142 .)
The current PET tracers, which are used for tumor diagnosis have some undisputed disadvantages: in FDG accumulates preferably in those cells with increased glucose metabolism on, but there are also other pathological and physiological conditions of increased glucose metabolism in the cells involved and tissues, eg, Ex. of infection or wound healing (summarized in J. Nucl. Med. Technol. (2005), 33, 145-155). It is still often difficult to decide whether a detected by FDG-PET lesion actually neoplastic origin or due to other physiological or pathological state of the tissue. Overall, the diagnostic activity by FDG-PET in oncology has a sensitivity of 84% and a specificity of 88% to(Gambhir et al., ” A tabulated summary of the FDG PET literature “J. Nucl. Med. 2001, 42, 1- 93S). Tumors in the brain can be represented very difficult in healthy brain tissue, for example, by the high accumulation of FDG.
The previously known 18 F-labeled amino acid derivatives are in some cases well suited to detect tumors in the brain ((review): Eur J Nucl Med Mol Imaging 2002 May; 29 (5):. 681-90), but they can in other tumors do not compete with the imaging properties of the “gold standard” [18 F] 2-FDG. The metabolic accumulation and retention of previously F-18 labeled amino acids in tumorous tissue is usually lower than for FDG. Moreover, the accessibility of isomerically pure F-18-labeled non-aromatic amino acids is chemically very demanding.
Similar to glucose increased metabolism in proliferating tumor cells has been described (Medina, J Nutr 1131: 2539S-2542S, 2001; Souba, Ann Surg 218:. 715-728, 1993) for glutamic acid and glutamine. The increased rate of protein and nucleic acid synthesis and energy production per se be accepted as reasons for increased Glutaminkonsum of tumor cells. The synthesis of the corresponding C-11 and C-14 labeled with the natural substrate thus identical compounds, has already been described in the literature (eg. Ex.Antoni, enzymes Catalyzed Synthesis of L- [4-C-11] Aspartate and L – [5-C-11] Glutamate J. Labelled Compd Radiopharm 44; (4) 2001: 287-294) and Buchanan, The biosynthesis of showdomycin: studies with stable isotopes and the determination of principal precursor J….. Chem. Soc. Chem. Commun .; EN; 22; 1984, 1515-1517). First indications with the C-11 labeled compound indicate no significant tumor accumulation.
Although the growth and proliferation of most tumors is fueled by glucose, some tumors are more likely to metabolize glutamine. In particular, tumor cells with the upregulated c-Myc gene are generally reprogrammed to utilize glutamine. We have developed new 3-fluoropropyl analogs of glutamine, namely [(18)F](2S,4R)- and [(18)F](2S,4S)-4-(3-fluoropropyl)glutamine, 3 and 4, to be used as probes for studying glutamine metabolism in these tumor cells. Optically pure isomers labeled with (18)F and (19)F (2S,4S) and (2S,4R)-4-(3-fluoropropyl)glutamine were synthesized via different routes and isolated in high radiochemical purity (≥95%). Cell uptake studies of both isomers showed that they were taken up efficiently by 9L tumor cells with a steady increase over a time frame of 120 min. At 120 min, their uptake was approximately two times higher than that of l-[(3)H]glutamine ([(3)H]Gln). These in vitro cell uptake studies suggested that the new probes are potential tumor imaging agents. Yet, the lower chemical yield of the precursor for 3, as well as the low radiochemical yield for 3, limits the availability of [(18)F](2S,4R)-4-(3-fluoropropyl)glutamine, 3. We, therefore, focused on [(18)F](2S,4S)-4-(3-fluoropropyl)glutamine, 4. The in vitro cell uptake studies suggested that the new probe, [(18)F](2S,4S)-4-(3-fluoropropyl)glutamine, 4, is most sensitive to the LAT transport system, followed by System N and ASC transporters. A dual-isotope experiment using l-[(3)H]glutamine and the new probe showed that the uptake of [(3)H]Gln into 9L cells was highly associated with macromolecules (>90%), whereas the [(18)F](2S,4S)-4-(3-fluoropropyl)glutamine, 4, was not (<10%). This suggests a different mechanism of retention. In vivo PET imaging studies demonstrated tumor-specific uptake in rats bearing 9L xenographs with an excellent tumor to muscle ratio (maximum of ∼8 at 40 min). [(18)F](2S,4S)-4-(3-fluoropropyl)glutamine, 4, may be useful for testing tumors that may metabolize glutamine related amino acids.
http://pubs.acs.org/doi/full/10.1021/mp500236y
ACS AuthorChoice – This is an open access article published under an ACS AuthorChoice License, which permits copying and redistribution of the article or any adaptations for non-commercial purposes.
Although the growth and proliferation of most tumors is fueled by glucose, some tumors are more likely to metabolize glutamine. In particular, tumor cells with the upregulated c-Myc gene are generally reprogrammed to utilize glutamine. We have developed new 3-fluoropropyl analogs of glutamine, namely [18F](2S,4R)- and [18F](2S,4S)-4-(3-fluoropropyl)glutamine, 3 and 4, to be used as probes for studying glutamine metabolism in these tumor cells. Optically pure isomers labeled with 18F and 19F (2S,4S) and (2S,4R)-4-(3-fluoropropyl)glutamine were synthesized via different routes and isolated in high radiochemical purity (≥95%). Cell uptake studies of both isomers showed that they were taken up efficiently by 9L tumor cells with a steady increase over a time frame of 120 min. At 120 min, their uptake was approximately two times higher than that of l-[3H]glutamine ([3H]Gln). These in vitro cell uptake studies suggested that the new probes are potential tumor imaging agents. Yet, the lower chemical yield of the precursor for 3, as well as the low radiochemical yield for 3, limits the availability of [18F](2S,4R)-4-(3-fluoropropyl)glutamine, 3. We, therefore, focused on [18F](2S,4S)-4-(3-fluoropropyl)glutamine, 4. The in vitro cell uptake studies suggested that the new probe, [18F](2S,4S)-4-(3-fluoropropyl)glutamine, 4, is most sensitive to the LAT transport system, followed by System N and ASC transporters. A dual-isotope experiment using l-[3H]glutamine and the new probe showed that the uptake of [3H]Gln into 9L cells was highly associated with macromolecules (>90%), whereas the [18F](2S,4S)-4-(3-fluoropropyl)glutamine, 4, was not (<10%). This suggests a different mechanism of retention. In vivo PET imaging studies demonstrated tumor-specific uptake in rats bearing 9L xenographs with an excellent tumor to muscle ratio (maximum of ∼8 at 40 min). [18F](2S,4S)-4-(3-fluoropropyl)glutamine, 4, may be useful for testing tumors that may metabolize glutamine related amino acids.
PATENT
US 20100290991
http://www.google.com/patents/US20100290991
PATENT
WO 2009141091
PATENT
http://www.google.co.ug/patents/EP2123621A1?cl=en
REFERENCES
Molecular Pharmaceutics (2014), 11(11), 3852-3866
| EP1923382A1 * | 18 Nov 2006 | 21 May 2008 | Bayer Schering Pharma Aktiengesellschaft | [18F] labelled L-glutamic acid, [18F] labelled glutamine, their derivatives, their use and processes for their preparation |
| FR1461184A | Title not available | |||
| JPS58113142A | Title not available | |||
| WO2008052788A1 * | 30 Oct 2007 | 8 May 2008 | Bayer Schering Pharma Aktiengesellschaft | [f-18]-labeled l-glutamic acid, [f-18]-labeled l-glutamine, derivatives thereof and use thereof and processes for their preparation |
////////
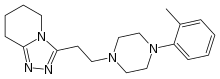
Dapiprazole
CAS 72822-12-9
HCL SALT 72822-13-0
| 5,6,7,8-Tetrahydro-3-(2-(4-(O-tolyl)-1-piperazinyl)ethyl)-S-triazolo(4,3-a)pyridine |
Dapiprazole (Rev-Eyes) is an alpha blocker. It is used to reverse mydriasis after eye examination.[1]
Used in the treatment of iatrogenically induced mydriasis produced by adrenergic (phenylephrine) or parasympatholytic (tropicamide) agents used in certain eye examinations.
Dapiprazole is an alpha-adrenergic blocking agent. It produces miosis by blocking the alpha-adrenergic receptors on the dilator muscle of the iris. Dapiprazole produces no significant action on ciliary muscle contraction and thus, there are no changes in the depth of the anterior chamber of the thickness of the lens. It does not alter the IOP either in normal eyes or in eyes with elevated IOP. The rate of pupillary constriction may be slightly slower in clients with brown irises than in clients with blue or green irises.
Dapiprazole acts through blocking the alpha1-adrenergic receptors in smooth muscle. It produces miosis through an effect on the dilator muscle of the iris and does not have any significant activity on ciliary muscle contraction and, therefore does not induce a significant change in the anterior chamber depth or the thickness of the lens.
Oral LD50 is 1189-2100 mg/kg in mice, rats and rabbits.

| Salt | ATC | formula | MM | CASE |
|---|---|---|---|---|
| – | N05AX S01EX02 |
C19H27N5 | 325.46 g / mol | 72822-12-9 |
| monogïdroxlorïd | N05AX S01EX02 |
C19H27N5 · HCl | 361.92 g / mol | 72822-13-0 |
 |
|
| Scheme illustration:By cyclization of O-methylvalerolactam (I) with 3-(4-o-tolyl-1-piperazinyl) propionic acid hydrazide (II) in refluxing xylene, followed by a treatment with ethanolic HCl. |
Acylation of (1-methylcyclopropyl)guanidine (IV) with 3-bromo-5-chlorothiophene-2-sulfonyl chloride (III) under Schotten-Baumann conditions afforded the sulfonyl guanidine (V). This was cyclized to the desired thienothiadiazine upon treatment with Cs2CO3 and Cu2O in boiling butanol.

In a different method, (1-methylcyclopropyl)guanidine (I) is acylated by 3-bromo-5-chlorothiophene-2-sulfonyl chloride (II) to produce the sulfonyl guanidine (III). Intramolecular cyclization of (III) in the presence of Cu2O and Cs2CO3 leads to the title thienothiadiazine derivative. Similarly, acylation of guanidine (I) with 3,5-dichlorothiophene-2-sulfonyl chloride (IV) provides sulfonyl guanidine (V), which is then cyclized in the presence of Cu2O and Cs2CO3.

In an alternative method, sulfonylation of N-isopropylguanidine (V) with 2,5-dichlorothiophene-3-sulfonyl chloride (IV) produced the sulfonyl guanidine (VI). This was then cyclized to the title compound by treatment with copper bronze and potassium carbonate in boiling DMF……..WO 0102410
| country | Tradename | Manufacturer |
|---|---|---|
| Germany | Remidrial | winegrower |
| Italy | Glamidolo | Angelini, 1987 |
| Ukraine | no | no |
 |
|
| Systematic (IUPAC) name | |
|---|---|
|
3-{2-[4-(2-methylphenyl)piperazin-1-yl]ethyl}-5,6,7,8-
tetrahydro-[1,2,4]triazolo[4,5-a]pyridine |
|
| Clinical data | |
| AHFS/Drugs.com | Consumer Drug Information |
| MedlinePlus | a601043 |
| Pregnancy category |
|
| Routes of administration |
Topical (eye drops) |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | Negligible when administered topically |
| Identifiers | |
| CAS Number | 72822-12-9  |
| ATC code | S01EX02 (WHO) |
| PubChem | CID 3033538 |
| IUPHAR/BPS | 7155 |
| DrugBank | DB00298  |
| ChemSpider | 2298190  |
| UNII | 5RNZ8GJO7K  |
| KEGG | D07775  |
| ChEBI | CHEBI:51066  |
| ChEMBL | CHEMBL1201216  |
| Chemical data | |
| Formula | C19H27N5 |
| Molar mass | 325.451 g/mol |
//////Дапипразол , Dapiprazole, AF-2139, Remydrial, Rev-Eyes, Reversil, Glamidolo
n1nc(n2c1CCCC2)CCN4CCN(c3ccccc3C)CC4
DRUG REGULATORY AFFAIRS INTERNATIONAL

For most companies manufacturing APIs and pharmaceutical products, the implementation of ICH Q3D has a serious impact – as shown in a survey recently carried out by the ECA. Read more about the issues encountered by many companies regarding the assessment and control of elemental impurities and the kind of support they wish.
SEE
One and a half years after the official entry into force of the ICH Q3D Guideline for “Elemental Impurities” and several supporting documents from the ICH (e.g. “Training Package: Modules 0-7“) a number of questions as regards implementation remain.
In a survey recently performed by the ECA, questions were posed about the issues relating to the fulfilling of the requirements laid down in ICH Q3D. The feedback from almost 80 participants from medium and large pharmaceutical companies and API manufacturers located in Germany and other EU Member States shows remarkable results which harsh…
View original post 247 more words
DRUG REGULATORY AFFAIRS INTERNATIONAL

EDQM’s new Guideline on Electronic Submissions for CEP Applications
As of today (June, 1st 2016), the EDQM doesn’t accept any CEP application in paper format. Read more here about the structure of the electronic submission of an application for a Certificate of Suitability and the errors to avoid.
SEE
The EDQM has recently published a document entitled “Guidance for electronic submissions for Certificates of Suitability (CEP) applications” (PA/PH/CEP (09) 108, 3R) in which the authority describes the requirements to be considered for the submission of an application for a CEP. Let us give you the most important message straight away: the EDQM now only accepts CEP applications in the electronic format since June 1st 2016.
Only the following formats are authorised within an application procedure: PDF, NeeS (non-eCTD electronic submission), VNeeS (the respective application format for veterinary purposes) and eCTD. A change of format during an ongoing…
View original post 320 more words

Ladostigil, TV-3,326
(N-propargyl-(3R) aminoindan-5yl)-ethyl methyl carbamate
(3R)-3-(Prop-2-ynylamino)indan-5-yl ethyl(methyl)carbamate; R-CPAI
Carbamic acid, ethylmethyl-, (3R)-2,3-dihydro-3-(2-propynylamino)-1H-inden-5-yl ester
Condition(s): Mild Cognitive Impairment
U.S. FDA Status: Mild Cognitive Impairment (Phase 2)
Company: Avraham Pharmaceuticals Ltd
Target Type: Cholinergic System
| CAS No: | 209349-27-4 |
|---|---|
| Synonyms: | Ladostigil, TV-3326, UNII-SW3H1USR4Q |
| Molecular Weight: | 272.346 g/mol |
| Chemical Formula: | C16-H20-N2-O2 |
| IUPAC Name: | (3R)-3-(Prop-2-ynylamino)indan-5-yl ethyl(methyl)carbamate N-Propargyl-(3R)-aminoindan-5-yl) ethyl methyl carbamate |

CAS 209394-46-7, Ladostigil tartrate
N-Ethyl-N-methylcarbamic acid 3(R)-(2-propynylamino)-2,3-dihydro-1H-inden-5-yl ester L-tartrate
In 2010, ladostigil tartrate was licensed by Technion Research & Development Foundation and Yissum to Avraham for the treatment of Alzheimer’s disease and other neurogenerative diseases.
Ladostigil (TV-3,326) is a novel neuroprotective agent being investigated for the treatment of neurodegenerative disorders likeAlzheimer’s disease, Lewy body disease, and Parkinson’s disease.[1] It acts as a reversible acetylcholinesterase andbutyrylcholinesterase inhibitor, and an irreversible monoamine oxidase B inhibitor, and combines the mechanisms of action of older drugs like rivastigmine and rasagiline into a single molecule.[2][3] In addition to its neuroprotective properties, ladostigil enhances the expression of neurotrophic factors like GDNF and BDNF, and may be capable of reversing some of the damage seen in neurodegenerative diseases via the induction of neurogenesis.[4] Ladostigil also has antidepressant effects, and may be useful for treating comorbid depression and anxiety often seen in such diseases as well.[5][6]
Ladostigil [(N-propargyl-(3R) aminoindan-5yl)-ethyl methyl carbamate] is a dual acetylcholine-butyrylcholineesterase and brain selective monoamine oxidase (MAO)-A and -B inhibitor in vivo (with little or no MAO inhibitory effect in the liver and small intestine), intended for the treatment of dementia co-morbid with extrapyramidal disorders and depression (presently in a Phase IIb clinical study). This suggests that the drug should not cause a significant potentiation of the cardiovascular response to tyramine, thereby making it a potentially safer antidepressant than other irreversible MAO-A inhibitors. Ladostigil was shown to antagonize scopolamine-induced impairment in spatial memory, indicating that it can cause significant increases in rat brain cholinergic activity. Furthermore, ladostigil prevented gliosis and oxidative-nitrative stress and reduced the deficits in episodic and spatial memory induced by intracerebroventricular injection of streptozotocin in rats. Ladostigil was demonstrated to possess potent anti-apoptotic and neuroprotective activities in vitro and in various neurodegenerative rat models, (e.g. hippocampal damage induced by global ischemia in gerbils and cerebral oedema induced in mice by closed head injury). These neuroprotective activities involve regulation of amyloid precursor protein processing; activation of protein kinase C and mitogen-activated protein kinase signaling pathways; inhibition of neuronal death markers; prevention of the fall in mitochondrial membrane potential and upregulation of neurotrophic factors and antioxidative activity. Recent findings demonstrated that the major metabolite of ladostigil, hydroxy-1-(R)-aminoindan has also a neuroprotective activity and thus, may contribute to the overt activity of its parent compound. This review will discuss the scientific evidence for the therapeutic potential use of ladostigil in Alzheimer’s and Lewy Body diseases and the molecular signaling pathways that are considered to be involved in the biological activities of the drug
Recently, Yissum Research Development Company associated with the Hebrew University of Jerusalem announced that the University will use a portion of a $9 million dollar grant awarded to Avraham Pharmaceuticals, Pontifax, Clal Biotechnology Industries and Professor Marta Weinstock-Rosin to complete the Phase II efficacy trial in patients afflicted with Alzheimer’s disease.
The trial will be conducted over the course of 52 weeks and will involve the novel drug, Ladostigil. Ladostigil is a new comprehensive drug used to combat symptoms of Alzheimer’s, Parkinson’s, depression, and anxiety. The drug is deemed multi-functional because it addresses a host of neurodegenerative problems. Ladostigil is a brain-selective monoamine oxidase inhibitor (MAOI) that protects the neurons.
US 20140243544 http://www.google.co.in/patents/US20140243544
WO 2008074816
http://www.google.com/patents/WO2008074816A1?cl=en
The procedure of example 7a is repeated with (S)-ethyl-methyl-carbamic acid 3-hydroxy-indan-5-yl ester instead of (R)-ethyl-methyl-carbamic acid 3-hydroxy-indan-5-yl ester. The R-enantiomer is produced.
Tetrahedron: Asymmetry (2012), 23(5), 333-338
http://www.sciencedirect.com/science/article/pii/S0957416612001334

 (R)-3-(Prop-2-ynylamino)-2,3-dihydro-1H-inden-5-yl ethyl(methyl)carbamate (R)-3-(Prop-2-ynylamino)-2,3-dihydro-1H-inden-5-yl ethyl(methyl)carbamate
C16H20N2O2 |
ee: 89% Source of chirality: the precursor Absolute configuration: (R) |
 (R)-3-(Prop-2-ynylamino)-2,3-dihydro-1H-inden-5-yl ethyl(methyl)carbamate hemi((2R,3R)-2,3-dihydroxysuccinate) (R)-3-(Prop-2-ynylamino)-2,3-dihydro-1H-inden-5-yl ethyl(methyl)carbamate hemi((2R,3R)-2,3-dihydroxysuccinate)
C36H46N4O10 |
ee: 99% Source of chirality: the precursor Absolute configuration: (R,R,R) |
Yavne, Israel, May 17, 2012 — Avraham Pharmaceuticals Ltd. announced today the commencement of a Phase 2 clinical trial to evaluate the safety and efficacy of ladostigil in patients diagnosed with mild cognitive impairment (MCI). This 36-month, multi-centre, randomized, double-blind, placebo-controlled trial will include at least 200 patients in 16 centers in Europe and Israel.
In parallel, Avraham Pharmaceuticals has also completed the enrollment of 200 patients in a Phase 2 trial of ladostigil, a novel molecule for the treatment of mild to moderate Alzheimer’s disease. The Phase 2 study is a double-blind, closed-label, placebo-controlled trial taking place at 20 sites in five countries across Europe. In January 2012, the Company performed an interim analysis of this Phase 2 trial, which indicated that the drug is safe and well tolerated, as well as shows a positive trend toward efficacy. Final results of the 26-week trial are expected in the fourth quarter of 2012.
The Company also announced today that it has appointed Yaacov Michlin, CEO of Yissum Research Development Company of the Hebrew University of Jerusalem Ltd., the technology transfer arm of the University, as Chairman of the Board and Yona Geffen, Ph.D., as Chief Executive Officer.
“We strongly believe in ladostigil and are confident that Yona’s background and extensive experience in developing therapies for neurological disorders and neurodegenerative diseases renders her the perfect choice to lead Avraham,” said Yaacov Michlin, Chairman of Avraham Pharmaceuticals and Chief Executive Officer of Yissum. “In further researches performed by Prof. Weinstock-Rosin, ladostigil has showed promise also for the treatment of MCI in addition to Alzheimer’s disease. We are pleased that another Phase 2 clinical trial in patients with MCI has begun in parallel, and look forward to the final results of the Phase 2 study for the treatment of Alzheimer’s disease expected at the end of this year.”
“I am delighted to lead Avraham in these exciting times for the company, as we advance ladostigil in 2 Phase 2 clinical trials simultaneously. We believe that this unique drug candidate has the potential to transform the treatment of various neurodegenerative diseases,” said Dr. Yona Geffen, Avraham Pharmaceuticals Chief Executive Officer.
Dr. Yona Geffen joined Avraham Pharmaceuticals in January 2011 as Senior Vice President of Clinical Affairs and Chief Operating Officer. Dr. Geffen has more than 12 years of experience in the field of drug development in biopharmaceutical companies. Prior to Avraham, she was Executive Drug Development Director at BiolineRx (NASDAQ: BLRX). Before that, Dr. Geffen was a project manager at Proneuron Biotechnologies. Dr. Geffen received her Ph.D. from Ben Gurion University in Beer Sheva, Israel. She also holds an M.Sc. in business management.
Yaacov Michlin has been CEO of Yissum since 2009. Prior to Yissum, Mr. Michlin spent over a decade in leading and assisting pharmaceutical, hi-tech and biomedical companies in various technology commercialization deals, licensing agreements, capital raising activities, partnerships, mergers and acquisitions. Michlin holds a Bachelor of Law and Economics cum laude, and a Master of Law all from Bar-Ilan University, Ramat Gan, Israel. In addition, he has an MBA cum laude from the Technion Israel Institute of Technology, Haifa, Israel.
About Ladostigil
Ladostigil is a novel cholinesterase and brain-selective monoamine oxidase inhibitor, and neuroprotective agent for the treatment of Alzheimer’s disease, mild cognitive impairment and other neurodegenerative diseases. The drug, which was exclusively licensed to Avraham Pharmaceuticals by Yissum Research Development Company Ltd., and by the Technion Research and Development Foundation Ltd. (TRDF), has proven to be safe and well tolerated in Phase 1 and Phase 2 clinical trials. Like other cholinesterase inhibitors currently on the market, ladostigil targets symptomatic relief in Alzheimer’s disease patients. But unlike these drugs, ladostigil, which also causes brain selective inhibition of monoamine oxidase (MAO) provides the potential to improve the behavioral and psychological symptoms of dementia such as depression and anxiety. Moreover, ladostigil has the potential to slow progression of clinical symptoms of Alzheimer’s disease for sustained periods of time and to modify the pathology associated with the disease. In addition, the neuroprotective activity of ladostigil provides a drug candidate that may have the potential to slow progression to Alzheimer’s disease in patients diagnosed with MCI. This potential has been amply demonstrated in animal models, especially in studies of ageing rats.
Ladostigil was designed by Professor Marta Weinstock-Rosin of the Hebrew University of Jerusalem, inventor of Exelon® and Professor Moussa B.H. Youdim of the Technion Israel Institute of Technology, inventor of Azilect®. The drug substance was first synthesized by Professor Michael Chorev of the Hebrew University, who is now based at Harvard University. All three distinguished scientists act as scientific advisors to Avraham Pharmaceuticals.
About Alzheimer’s Disease
Alzheimer’s disease is the most common cause of dementia worldwide, affecting about one in 20 people 65 years of age or older, accounting for 60-80% of dementia cases. In 2010, 5.4 million people were affected by Alzheimer’s disease in the U.S., where it is the 6th leading cause of death. In Europe, more than 6 million are living with the disease. Approximately half of Alzheimer’s patients also suffer from depression, and up to 40% also exhibit Parkinson-like symptoms.
About Mild Cognitive Impairment
Mild cognitive impairment (MCI) is a syndrome defined as an intermediate stage between the expected cognitive decline of normal aging and the more pronounced decline of dementia. It involves problems with memory, language, thinking and judgment that are greater than typical age-related changes. Although MCI can present with a variety of symptoms, when memory loss is the predominant symptom it is termed “amnestic MCI” and is frequently seen as a prodromal stage of Alzheimer’s disease. Prevalence in population-based epidemiological studies ranges from 3% to 19% in adults older than 65 years. There is no proven treatment or therapy for MCI.
About Avraham Pharmaceutical
Founded in 2010, Avraham Pharmaceuticals has raised more than $12 million to advance the development of its unique, multi-functional drug substance, ladostigil, currently undergoing two Phase 2 clinical trials for the treatment of Alzheimer’s disease and mild cognitive impairment. The Company has been capitalized by Clal Biotechnology Industries Ltd., the Pontifax Fund and Yissum Research Development Company Ltd., the technology transfer arm of the Hebrew University.
Yona Geffen CEO
Avraham Pharmaceuticals Ltd.
42 Hayarkon st.
Northern Industrial Zone
Yavneh, 81227
Israel
| WO1998027055A1 * | 18 Dec 1997 | 25 Jun 1998 | Teva Pharmaceutical Industries, Ltd. | Aminoindan derivatives |
| WO2005051371A1 | 28 Sep 2004 | 9 Jun 2005 | Technion Research & Development Foundation Ltd. | Compositions and methods for treatment of cardiovascular disorders and diseases |
| WO2006130726A2 | 31 May 2006 | 7 Dec 2006 | Teva Pharmaceutical Industries, Ltd. | Use of ladostigil for the treatment of multiple sclerosis |
| WO2007087029A2 * | 11 Dec 2006 | 2 Aug 2007 | Yissum Research Development Company Of The Hebrew University Of Jerusalem | Use of low-dose ladostigil for neuroprotection |
| WO2009022345A1 | 14 Aug 2008 | 19 Feb 2009 | Yissum Research Development Company Of The Hebrew University Of Jerusalem | Phenyl carbamates for the treatment of multiple sclerosis |
| WO2009022346A2 | 14 Aug 2008 | 19 Feb 2009 | Yissum Research Development Company Of The Hebrew University Of Jerusalem | Phenyl carbamates for treating gastrointestinal inflammation |
| WO2012059920A1 | 2 Nov 2011 | 10 May 2012 | Yissum Research Development Company Of The Hebrew University Of Jerusalem Ltd. | Ladostigil dosage regime |
| US6251938 | 18 Jun 1999 | 26 Jun 2001 | Teva Pharmaceutical Industries, Ltd., | Phenylethylamine derivatives |
| US6303650 | 18 Jun 1999 | 16 Oct 2001 | Yissum Research Development Company Of The Hebrew University Of Jerusalem | Aminoindan derivatives |
| US6538025 | 31 Aug 2001 | 25 Mar 2003 | Teva Pharmaceutical Industries, Ltd. | Aminoindan derivatives |
| US7335685 | 22 Feb 2006 | 26 Feb 2008 | Teva Pharmaceutical Industries, Ltd. | Crystals of ladostigil tartrate, methods of production and pharmaceutical compositions thereof |
| US7375249 | 21 Feb 2006 | 20 May 2008 | Teva Pharmaceutical Industries Ltd. | Process for the synthesis of enantiomeric indanylamine derivatives |
| US7476757 | 15 Apr 2008 | 13 Jan 2009 | Teva Pharmaceutical Industries Ltd. | Process for the synthesis of enantiomeric indanylamine derivatives |
| US7491847 | 15 Nov 2006 | 17 Feb 2009 | Teva Pharmaceutical Industries, Ltd. | Methods for isolating propargylated aminoindans |
| US20050222123 | 27 Jan 2005 | 6 Oct 2005 | North Shore-Long Island Jewish Research Institute | Cholinesterase inhibitors for treating inflammation |
| US20060189685 | 24 Feb 2006 | 24 Aug 2006 | Daniella Licht | Formulations of ladostigil tartrate |
| US20060189819 | 22 Feb 2006 | 24 Aug 2006 | Teva Pharmaceutical Industries, Ltd. | Crystals of ladostigil tartrate, methods of production and pharmaceutical compositions thereof |
| US20060199974 | 21 Feb 2006 | 7 Sep 2006 | Teva Pharmaceutical Industries Ltd. | Process for the synthesis of enantiomeric indanylamine derivatives |
| US20070088082 | 28 Sep 2006 | 19 Apr 2007 | Judith Aronhime | Polymorphic forms of ladostigil tartrate |
| US20070093549 | 28 Sep 2006 | 26 Apr 2007 | Judith Aronhime | Methods for preparation of ladostigil tartrate crystalline form A1 |
| US20070112217 | 15 Nov 2006 | 17 May 2007 | Anton Frenkel | Methods for isolating propargylated aminoindans |
| US20070135518 | 8 Dec 2006 | 14 Jun 2007 | Marta Weinstock-Rosin | Use of low-dose ladostigil for neuroprotection |
| US20070203232 | 23 Feb 2007 | 30 Aug 2007 | Victor Piryatinsky | Propargylated aminoindans, processes for preparation, and uses thereof |
| US20070232691 | 28 Mar 2007 | 4 Oct 2007 | Tamar Goren | Use of ladostigil for the treatment of schizophrenia |
| US20070293583 | 11 Dec 2006 | 20 Dec 2007 | Marta Weinstock-Rosin | Use of low-dose ladostigil for neuroprotection |
| US5532415 * | Mar 28, 1995 | Jul 2, 1996 | Teva Pharmaceutical Industries Ltd. | R-enantiomer of N-propargyl-1-aminoindan, salts, compositions and uses thereof |
| US5703059 * | Jan 19, 1994 | Dec 30, 1997 | British Biotech Pharmaceuticals Ltd. | Disaccharide ligands for selectins |
| US5936000 * | Jan 16, 1996 | Aug 10, 1999 | Pharmacia & Upjohn Company | 2-aminoindans as selective dopamine D3 ligands |
| US6271261 * | Jun 24, 1997 | Aug 7, 2001 | Smithkline Beecham Corporation | IL-8 receptor antagonists |
| US6271263 * | Mar 2, 1999 | Aug 7, 2001 | Teva Pharmaceutical Industries, Ltd. | Compositions containing and methods of using 1-aminoindan and derivatives thereof and process for preparing optically active 1-aminoindan derivatives |
| US6303650 * | Jun 18, 1999 | Oct 16, 2001 | Yissum Research Development Company Of The Hebrew University Of Jerusalem | Aminoindan derivatives |
| US6462222 * | Aug 31, 2001 | Oct 8, 2002 | Yissum Research Development Company Of The Hebrew University Of Jerusalem | Aminoindan derivatives |
| US6538025 * | Aug 31, 2001 | Mar 25, 2003 | Teva Pharmaceutical Industries, Ltd. | Aminoindan derivatives |
| US6737547 * | Sep 15, 1999 | May 18, 2004 | Teva Pharmaceutical Industries, Ltd. | Compositions containing and methods of using N-acyl-1H-aminoindenes |
| US20040010038 * | Feb 27, 2003 | Jan 15, 2004 | Eran Blaugrund | Propargylamino indan derivatives and propargylamino tetralin derivatives as brain-selective MAO inhibitors |
| Citing Patent | Filing date | Publication date | Applicant | Title |
|---|---|---|---|---|
| US7649115 | Jun 1, 2006 | Jan 19, 2010 | Jenrin Discovery, Inc. | MAO-B inhibitors useful for treating obesity |
| US8541475 | Dec 31, 2009 | Sep 24, 2013 | Jenrin Discovery, Inc. | MAO-B inhibitors useful for treating obesity |
| US8569545 | Jun 2, 2009 | Oct 29, 2013 | Generics (Uk) Limited | Process for the preparation of enantiomerically pure amines |
| US8809589 | Jul 18, 2013 | Aug 19, 2014 | Generics [Uk] Limited | Process for the preparation of enantiomerically pure amines |
| US20070088004 * | Jun 1, 2006 | Apr 19, 2007 | Mcelroy John F | MAO-B inhibitors useful for treating obesity |
| US20100168068 * | Dec 31, 2009 | Jul 1, 2010 | Jenrin Discovery | Mao-b inhibitors useful for treating obesity |
| US20110184071 * | Jun 2, 2009 | Jul 28, 2011 | Vinayak Gore | process for the preparation of amines |
| US20110218360 * | Sep 8, 2011 | Dr. Reddy’s Laboratories Ltd. | Preparation of rasagiline and salts thereof | |
| CN103443111A * | Apr 2, 2012 | Dec 11, 2013 | 高砂香料工业株式会社 | Novel ruthenium complex and process for producing optically active alcohol compound using same as catalyst |
| CN103443111B * | Apr 2, 2012 | Mar 2, 2016 | 高砂香料工业株式会社 | 钌配合物以及以该配合物作为催化剂的光学活性醇化合物的制备方法 |
| WO2013118126A1 | Feb 11, 2013 | Aug 15, 2013 | Yissum Research Development Company Of The Hebrew University Of Jerusalem Ltd. | Ladostigil therapy for immunomodulation |
 |
|
| Systematic (IUPAC) name | |
|---|---|
|
[(3R)-3-(prop-2-ynylamino)indan-5-yl]-N-propylcarbamate
|
|
| Clinical data | |
| Routes of administration |
Oral |
| Legal status | |
| Legal status |
|
| Identifiers | |
| CAS Number | 209349-27-4 |
| ATC code | none |
| PubChem | CID 208907 |
| ChemSpider | 181005 |
| UNII | SW3H1USR4Q  |
| Synonyms | [N-propargyl-(3R)-aminoindan-5yl]-N-propylcarbamate |
| Chemical data | |
| Formula | C16H20N2O2 |
| Molar mass | 272.34 g/mol |
///////////Ladostigil, TV-3,326
c1c(cc2c(c1)CC[C@H]2NCC#C)OC(=O)N(CC)C

Obeticholic acid
Obeticholic acid; 6-ECDCA; INT-747; 459789-99-2; 6-Ethylchenodeoxycholic acid; 6alpha-Ethyl-chenodeoxycholic acid;
(4R)-4-[(3R,5S,6R,7R,8S,9S,10S,13R,14S,17R)-6-ethyl-3,7-dihydroxy-10,13-dimethyl-2,3,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl]pentanoic acid
| Molecular Formula: | C26H44O4 |
|---|---|
| Molecular Weight: | 420.62516 g/mol |
NDA Filed
A farnesoid X receptor (FXR) agonist potentially for treatment of primary biliary cirrhosis and nonalcoholic steatohepatitis.

6-ECDCA; DSP-1747; INT-747
CAS No.459789-99-2
Obeticholic acid (abbreviated to OCA), is a semi-synthetic bile acid analogue which has the chemical structure 6α-ethyl-chenodeoxycholic acid. It has also been known as INT-747. It is undergoing development as a pharmaceutical agent for severalliver diseases and related disorders. Intercept Pharmaceuticals Inc. (NASDAQ symbol ICPT) hold the worldwide rights to develop OCA outside Japan and China, where it is licensed to Dainippon Sumitomo Pharma.[2]
REVIEW
INT-747(Obeticholic acid; 6-ECDCA) is a potent and selective FXR agonist(EC50=99 nM) endowed with anticholestatic activity. IC50 value: 99 nM(EC50) [1] Target: FXR agonist in vitro: The exposure of rat hepatocytes to 1 microM 6-ECDCA caused a 3- to 5-fold induction of small heterodimer partner (Shp) and bile salt export pump (bsep) mRNA and 70 to 80% reduction of cholesterol 7alpha-hydroxylase (cyp7a1), oxysterol 12beta-hydroxylase (cyp8b1), and Na(+)/taurocholate cotransporting peptide (ntcp) [2]. in vivo: In vivo administration of 6-ECDCA protects against cholestasis induced by E(2)17alpha [2]. high salt (HS) diet significantly increased systemic blood pressure. In addition, HS diet downregulated tissue DDAH expression while INT-747 protected the loss in DDAH expression and enhanced insulin sensitivity compared to vehicle controls [3]. Rats were gavaged with INT-747 or vehicle during 10 days after bile-duct ligation and then were assessed for changes in gut permeability, BTL, and tight-junction protein expression, immune cell recruitment, and cytokine expression in ileum, mesenteric lymph nodes, and spleen. After INT-747 treatment, natural killer cells and interferon-gamma expression markedly decreased, in association with normalized permeability selectively in ileum (up-regulated claudin-1 and occludin) and a significant reduction in BTL [4].
REFERENCES
| [1] | Verbeke L, et al. The FXR Agonist Obeticholic Acid Prevents Gut Barrier Dysfunction and Bacterial Translocation in Cholestatic Rats. Am J Pathol. 2015 Feb;185(2):409-19. |
| [2] | Ghebremariam YT, et al. FXR agonist INT-747 upregulates DDAH expression and enhances insulin sensitivity in high-salt fed Dahl rats. PLoS One. 2013 Apr 4;8(4):e60653. |
| [3] | Fiorucci S, et al. Protective effects of 6-ethyl chenodeoxycholic acid, a farnesoid X receptor ligand, in estrogen-induced cholestasis. J Pharmacol Exp Ther. 2005 May;313(2):604-12. |
| [4] | Pellicciari R, et al. 6alpha-ethyl-chenodeoxycholic acid (6-ECDCA), a potent and selective FXR agonist endowed with anticholestatic activity. J Med Chem. 2002 Aug 15;45(17):3569-72. |
The natural bile acid, chenodeoxycholic acid, was identified in 1999 as the most active physiological ligand for the farnesoid X receptor (FXR), which is involved in many physiological and pathological processes. A series of alkylated bile acid analogues were designed, studied and patented by Roberto Pellicciari and colleagues at the University of Perugia, with 6α-ethyl-chenodeoxycholic acid emerging as the most highly potent FXR agonist.[3] FXR-dependent processes in liver and intestine were proposed as therapeutic targets in human diseases.[4] Obeticholic acid is the first FXR agonist to be used in human drug studies.

OCA is undergoing development in phase 2 and 3 studies for specific liver and gastrointestinal disorders.[5]
Primary biliary cirrhosis (PBC) is an auto-immune, inflammatory liver disease which produces bile duct injury, fibrosis, cholestasisand eventual cirrhosis. It is much more common in women than men and can cause jaundice, itching (pruritus) and fatigue.Ursodeoxycholic acid therapy is beneficial, but the disease often progresses and may require liver transplantation.[6] Animal studies suggested that treatment with FXR agonists should be beneficial in cholestatic diseases such as PBC.[7] OCA at doses between 10 mg and 50 mg was shown to provide significant biochemical benefit, but pruritus was more frequent with higher doses.[8][9] The results of a randomized, double-blind phase 3 study of OCA, 5 mg or 10 mg, compared to placebo (POISE) were presented in April 2014, and showed that the drug met the trial’s primary endpoint of a significant reduction in serum alkaline phosphatase, abiomarker predictive of disease progression, liver transplantation or death.[10]

Non-alcoholic steatohepatitis is a common cause of abnormal liver function with histological features of fatty liver, inflammation andfibrosis. It may progress to cirrhosis and is becoming an increasing indication for liver transplantation. It is increasing in prevalence. OCA is proposed to treat NASH.[11] A phase 2 trial published in 2013 showed that administration of OCA at 25 mg or 50 mg daily for 6 weeks reduced markers of liver inflammation and fibrosis and increased insulin sensitivity.[12]
The Farnesoid X Receptor Ligand Obeticholic Acid in Nonalcoholic Steatohepatitis Treatment (FLINT) trial, sponsored by NIDDK, was halted early in January 2014, after about half of the 283 subjects had completed the study, when a planned interim analysis showed that a) the primary endpoint had been met and b) lipid abnormalities were detected and arose safety concerns. Treatment with OCA (25 mg/day for 72 weeks) resulted in a highly statistically significant improvement in the primary histological endpoint, defined as a decrease in the NAFLD Activity Score of at least two points, with no worsening of fibrosis. 45% (50 of 110) of the treated group had this improvement compared with 21% (23 of 109) of the placebo-treated controls.[13] However concerns about longterm safety issues such as increased cholesterol and adverse cardiovascular events may warrant the concomitant use of statins in OCA-treated patients.[14]
Animal studies suggest that OCA improves intrahepatic vascular resistance and so may be of therapeutic benefit in portal hypertension.[15] An open label phase 2a clinical study is under way.
Bile acid diarrhea (also called bile acid malabsorption) can be secondary to Crohn’s disease or be a primary condition. Reduced median levels of FGF19, an ileal hormone that regulates increased hepatic bile acid synthesis, have been found in this condition.[16] FGF19 is potently stimulated by bile acids and especially by OCA.[17] A proof of concept study of OCA (25 mg/d) has shown clinical and biochemical benefit.[18]

Take 10g of austempered cholic acid 89.6% purity crude (single hetero greater than 2%), 3 times its weight of acetone and added to their 20% by weight of triethylamine was added, was heated at reflux for 2h, cooled slowly to 10 ° C, the precipitated crystals were filtered to give Obey acid organic amine salt crystals.
Acidification [0020] The organic amine salts Obey acid crystals were dissolved with purified water after 10wt% by mass percentage to the PH value of 2.0 with dilute hydrochloric acid, filtered and dried to give purified Obey acid.
[0021] The purified Obey acid ethyl acetate dissolved by heating and then cooling to 20 ° C, the precipitated crystals were filtered and dried to obtain a purity of 98.7% recrystallization Obey acid (single hetero less than 0.1%), recovery was 84.5%.
According to Obey acid 6 was prepared in the form of C Patent Document W02013192097A1 reaction of Example 1, as follows:
The 3 a – hydroxy -6 a – ethyl-7-keto -5 P – 24-oic acid (. 86g, 205 4mmol), water (688mL) and 50% (w / w) hydrogen sodium hydroxide solution (56. 4mL) and the mixture of sodium borohydride (7. 77g, 205. 4mmol) in a mixture of 50% (w / w) sodium hydroxide solution (1.5 mL of) and water (20 mL) in 90 ° in C to 105 ° C reaction. Was heated with stirring under reflux for at least 3 hours, the reaction was completed, the reaction solution was cooled to 80 ° C. Between 30 ° C at 50 ° C of citric acid (320. 2g, anhydrous), a mixture of n-butyl acetate (860 mL of) and water (491mL) to ensure an acidic pH of the aqueous phase was separated. Evaporation of the organic phase was distilled to give the residue was diluted with n-butyl acetate, slowly cooled to 15 ° C to 20 ° C, centrifugation. The crude product was crystallized from n-butyl acetate. After Obey acid isolated by n-butyl acetate (43mL, 4 times), dried samples were dried at 80 ° C under vacuum. To give 67. 34g (77. 9%) crystalline form C Obey acid.
| Patent ID | Date | Patent Title |
|---|---|---|
| US8546365 | 2013-10-01 | Bile acid derivatives as FXR ligands for the prevention or treatment of FXR-mediated diseases or conditions |
| US8377916 | 2013-02-19 | Steroids as agonists for FXR |
| US8058267 | 2011-11-15 | STEROIDS AS AGONISTS FOR FXR |
| US7994352 | 2011-08-09 | Process for Preparing 3a(Beta)-7a(Beta)-Dihydroxy-6a(Beta)-Alkyl-5Beta-Cholanic Acid |
| US7932244 | 2011-04-26 | Bile acid derivatives as FXR ligands for the prevention or treatment of FXR-mediated diseases or conditions |
| US7786102 | 2010-08-31 | Steroids as agonists for FXR |
| US2009062526 | 2009-03-05 | NOVEL METHOD OF SYNTHESIZING ALKYLATED BILE ACID DERIVATIVES |
| US7138390 | 2006-11-21 | Steroids as agonists for fxr |
| US2005107475 | 2005-05-19 | Methods of using farnesoid x receptor (frx) agonists |
| Patent ID | Date | Patent Title |
|---|---|---|
| US2016074419 | 2016-03-17 | Preparation and Uses of Obeticholic Acid |
| US2015359805 | 2015-12-17 | Bile Acid Derivatives as FXR Ligands for the Prevention or Treatment of FXR-Mediated Diseases or Conditions |
| US2015166598 | 2015-06-18 | Steroids as Agonists for FXR |
| US2014371190 | 2014-12-18 | Farnesoid X receptor modulators |
| US2014186438 | 2014-07-03 | COMPOSITIONS COMPRISING EPA AND OBETICHOLIC ACID AND METHODS OF USE THEREOF |
| US2014148428 | 2014-05-29 | Treatment of Pulmonary Disease |
| US2014057886 | 2014-02-27 | Bile Acid Derivatives as FXR Ligands for the Prevention or Treatment of FXR-Mediated Diseases or Conditions |
| US2014024631 | 2014-01-23 | Steroids as Agonists for FXR |
| US2013345188 | 2013-12-26 | Preparation and Uses of Obeticholic Acid |
| US8546365 | 2013-10-01 | Bile acid derivatives as FXR ligands for the prevention or treatment of FXR-mediated diseases or conditions |
 |
|
| Systematic (IUPAC) name | |
|---|---|
|
(3α,5β,6α,7α)-6-Ethyl-3,7-dihydroxycholan-24-oic acidOR (4R)-4-[(3R,5S,6R,7R,8S,9S,10S,13R,14S,17R)-6-ethyl-3,7-dihydroxy-10,13-dimethyl-2,3,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl]pentanoic acid |
|
| Clinical data | |
| Routes of administration |
Oral |
| Legal status | |
| Legal status |
|
| Identifiers | |
| CAS Number | 459789-99-2 |
| ATC code | A05AA04 (WHO) |
| PubChem | CID 447715 |
| IUPHAR/BPS | 3435 |
| ChemSpider | 394730 |
| UNII | 0462Z4S4OZ |
| KEGG | C15636 |
| ChEMBL | CHEMBL566315 |
| Synonyms | 6α-ethyl-chenodeoxycholic acid; INT-747 |
| Chemical data | |
| Formula | C26H44O4 |
| Molar mass | 420.62516 g/mol |
/////////6-ECDCA, DSP-1747, INT-747, 459789-99-2, Obeticholic acid
CC[C@@H]1[C@@H]2C[C@@H](CC[C@@]2([C@H]3CC[C@]4([C@H]([C@@H]3[C@@H]1O)CC[C@@H]4[C@H](C)CCC(=O)O)C)C)O
CCC1C2CC(CCC2(C3CCC4(C(C3C1O)CCC4C(C)CCC(=O)O)C)C)O
AM 2394
1-(6′-(2-hydroxy-2-methylpropoxy)-4-((5-methylpyridin-3-yl)oxy)-[3,3′-bipyridin]-6-yl)-3-methylurea
Urea, N-[6′-(2-hydroxy-2-methylpropoxy)-4-[(5-methyl-3-pyridinyl)oxy][3,3′-bipyridin]-6-yl]-N‘-methyl-
CAS 1442684-77-6
Chemical Formula: C22H25N5O4
Exact Mass: 423.1907
Array Biopharma Inc., Amgen Inc. INNOVATORS
AM-2394 is a potent and selective Glucokinase agonist (GKA), which catalyzes the phosphorylation of glucose to glucose-6-phosphate. AM-2394 activates GK with an EC50 of 60 nM, increases the affinity of GK for glucose by approximately 10-fold, exhibits moderate clearance and good oral bioavailability in multiple animal models, and lowers glucose excursion following an oral glucose tolerance test in an ob/ob mouse model of diabetes
Type 2 diabetes mellitus (T2DM) is a disease characterized by elevated plasma glucose in the presence of insulin resistance and inadequate insulin secretion. Glucokinase (GK), a member of the hexokinase enzyme family, catalyzes the phosphorylation of glucose to glucose-6-phosphate in the presence of ATP.

Glucokioase i exok ase IV or D> is a glycolytic enssyiris that plays, an importaat. role irt blood sugar regulation .related to glucose utifeattoti a»d metabolism in the liver and pancreatic beta •cells. Serving as a glucose sessor, gtoeokiuase controls lasma glucose, levels. Glucokinaae plays a doal rob in .reducing plasma glucose levels; glucose-mediated activation of the en¾ymc in hepatocytes facilitates hepatic giocose npiafcc aad glycogen synthesis, while that la pancreatic beta ceils ultimately induces ins lin seeretio«. Both of these effects in turn reduce plasma glucose levels.
Clinical evidence has shown that, glueokitiase variants with, decreased, and increased activities are associated with mature easel, diabetes of the y ung { O0Y2) and persistent: hyperinsul nemic hypoglycemia &( infancy (PHHI), respectively. lso, aoo n.sulin dependent diabetes rneilitos (NIDDM) patients have been reported to have inappropriately lo giueokaiase activity; Ftirtherrnare. overexpressioa of glucokiuase it* dietary or gesetie animal models of diabetes either prevents, aoKiiorafes, or reverses the progress of pathological. symptoms in the disease. For these reasons, compounds that activate gfecokiaase have been sought by the pitasaaceatjeai liidustry.
International patent application, Publication No. WO 2 7/OS3345, which was published on May 10, 200?, discloses as giocokinase act ators certain 2-an«.aopyridiiie derivatives bearing at the 3 -position a meihyieneoxy-dkrked aromatic group a d on. the ammo group a heteroaryl ring, such as dna/oly! or i A4-lmadiazoiyl
it has .now been found that pyridyl ureas are useful as glneokirtase activators. Cettain of these •compounds have been, found to have an outstanding combination of properties that especially adapts them, for oral use to control plasma glucose levels.


http://pubs.acs.org/doi/abs/10.1021/acsmedchemlett.6b00140
Glucokinase (GK) catalyzes the phosphorylation of glucose to glucose-6-phosphate. We present the structure–activity relationships leading to the discovery of AM-2394, a structurally distinct GKA. AM-2394 activates GK with an EC50 of 60 nM, increases the affinity of GK for glucose by approximately 10-fold, exhibits moderate clearance and good oral bioavailability in multiple animal models, and lowers glucose excursion following an oral glucose tolerance test in an ob/ob mouse model of diabetes.
WO 2013086397
Example. 1734 t¾^Jtiyi¾rea
Stirred vig rousl in f 0% MeOH irt EtOAc arid die res dtipg solid was colleeied. via vactiiars fiirfati m.
Trie two batches wen i coiiibiaed to yield I-(5-bmmo-4 5^»ie†fey pyiidin-3-yl xy)p Tidin-2- d 3~ metbySurea (I S J g, 5 3.7 om»)i, 76% yield).
S e .8: In 2 niL ofc ioxane
yI) iyridMJ-2-yios:y)pf¾ps3i-2-oI (0,098 g, 0.33 «ΜΠΟΪ), “ -i5-bs¾tao-4-{5-a3fidiy I py f idia-3 – ylosy)f5yridia-2-yl)-3-raethyl«rea (0.075 g, 0.22 tn ol.. t, and.2M poiass.ua» carbonate (0.33 ml, 0.67 m oi} artd tfets was s parged wi h At .for 10 mia before PdC§4dppl)*DCM (0.01 g g, 0.022 msttol) was added and dre reae!io a was sparged for aaotber 5 ma-, ir efore a was sealed and heated to 100 oversight The react! art was then loaded directly onto s ilica gel (50% acetone to PCM w4i. }%
MH40H) to afford i – (6′-(2diydioxy-2i-H5eth:ylpropCis:y) -4-{ 5″i:t re th y Ipy r i d i rt -3- io s y ) -3 ,3 : -bipyr id i rt -6- yl)-3-aie5¾ylt)rea φ.? 42 , 0.096 m ol, 43 % yield). !1 1 HMR (400 Mife, CDCij) 3 ppm 9.06 is,. !H),
S.33 is, 1H>, 8,27 (rs 2H), 8. Π (s, I H): K. (s, IHU 82 (dd, j-S.fi, 5.9 H HI), 1.21 (S !H), 6,«8
(d, Hz, i i i ). 6. ,4 (s:. m>, 4.25 (s, 2H), 2,87 (dj =4,3 Hz„ 3H) 2,37 (s, 3H>. 1 .33 is, <SH). Mass speetram (apci) tar/, : – 423.9 (M÷H).
Novel Series of Potent Glucokinase Activators Leading to the Discovery of AM-2394
Paul J. Dransfield, Vatee Pattaropong, Sujen Lai, Zice Fu, Todd J. Kohn, Xiaohui Du, Alan Cheng, Yumei Xiong, Renee Komorowski, Lixia Jin, Marion Conn, Eric Tien, Walter E. DeWolf Jr., Ronald J. Hinklin, Thomas D. Aicher, Christopher F. Kraser, Steven A. Boyd, Walter C. Voegtli, Kevin R. Condroski, Murielle Veniant-Ellison, Julio C. Medina, Jonathan Houze, and Peter Coward
Publication Date (Web): May 23, 2016 (Letter)
DOI: 10.1021/acsmedchemlett.6b00140
/////////Glucokinase activator, GKA, AM-2394, 1442684-77-6, AM 2394, Amgen
O=C(NC)NC1=CC(OC2=CC(C)=CN=C2)=C(C3=CC=C(OCC(C)(O)C)N=C3)C=N1

New and novel anti-norovirus agents
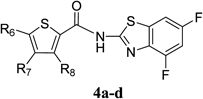
There is an urgent need for structurally novel anti-norovirus agents. In this study, we describe the synthesis, anti-norovirus activity, and structure–activity relationship (SAR) of a series of heterocyclic carboxamide derivatives. Heterocyclic carboxamide 1 (50% effective concentration (EC50)=37 µM) was identified by our screening campaign using the cytopathic effect reduction assay. Initial SAR studies suggested the importance of halogen substituents on the heterocyclic scaffold and identified 3,5-di-boromo-thiophene derivative 2j (EC50=24 µM) and 4,6-di-fluoro-benzothiazole derivative 3j (EC50=5.6 µM) as more potent inhibitors than 1. Moreover, their hybrid compound, 3,5-di-bromo-thiophen-4,6-di-fluoro-benzothiazole 4b, showed the most potent anti-norovirus activity with a EC50 value of 0.53 µM (70-fold more potent than 1). Further investigation suggested that 4b might inhibit intracellular viral replication or the late stage of viral infection.
According to the same procedure used for 2f, starting from 3,5-dibromothiophene-2-carboxylic acid (286 mg, 1.00 mmol) and 4,6-difluorobenzo[d]thiazol-2-amine (204 mg, 1.10 mmol), 4b (270 mg, 60%) was obtained as white powder. mp: 245–246°C. 1H-NMR (DMSO-d6) δ: 7.43 (1H, dt, J=10.2, 2.0 Hz), 7.56 (1H, s), 7.83 (1H, dd, J=8.4, 2.0 Hz). 13C-NMR (DMSO-d6) δ: 102.2 (dd, J=28.0, 23.1 Hz), 104.7 (dd, J=26.4, 3.3 Hz), 114.3, 118.4, 131.4 (d, J=7.4 Hz), 134.3 (d, J=10.7 Hz), 134.9, 135.2, 152.7 (d, J=241.2, 20.7 Hz), 158.3 (dd, J=242.2, 10.7 Hz), 159.0, 159.7. HPLC purity: >99%, ESI-MS m/z 453 [M+H]+.
Antiviral Activity and Cytotoxicity of Tetra-halogenated Hybrid Compounds
 |
|||||
|---|---|---|---|---|---|
| Compound | R6 | R7 | R8 | EC50 (µM)a) | CC50 (µM)b) |
| 4a | Cl | H | H | 2.1 | >100 |
| 4b | Br | H | Br | 0.53 | >100 |
| 4c | Cl | H | Cl | 1.1 | >100 |
| 4d | Cl | Cl | H | 1.4 | 31 |
a) EC50 was evaluated by the CPE reduction assay. 280 TCID50/50 µL of MNV and a dilution series of each compound were incubated for 30 min. The mixture was exposed to RAW264.7 cells for 1 h (in duplicate). b) Cytotoxicity was evaluated by the WST-8 assay. RAW264.7 cells were treated with dilution series of each compound (in triplicate) for 72 h.
Mai Ohba1) 2), Tomoichiro Oka3), Takayuki Ando1) 2), Saori Arahata4), Asaka Ikegaya4), Hirotaka Takagi5), Naohisa Ogo1) 2), Kazuhiro Owada2), Fumihiko Kawamori4), Qiuhong Wang6), Linda J. Saif6), Akira Asai1)
1) Center for Drug Discovery, Graduate School of Pharmaceutical Sciences, University of Shizuoka 2) Department of Pharmaceutical and Food Science, Shizuoka Institute of Environment and Hygiene 3) Department of Virology II, National Institute of Infectious Diseases 4) Department of Microbiology, Shizuoka Institute of Environment and Hygiene 5) Division of Biological Safety Control and Research, National Institute of Infectious Diseases 6) Food Animal Health Research Program, Ohio Agricultural Research and Development Center, Department of Veterinary Preventive Medicine, College of Veterinary Medicine, The Ohio State University

Three Methods:Killing Norovirus with Good HygieneKilling Norovirus in Your HomeTreating NorovirusCommunity Q&A
Norovirus is a contagious virus that affects many people each year. You can get norovirus through interaction with an infected person, by eating contaminated food, touching contaminated surfaces, or drinking contaminated water. However, there are ways to kill norovirus before it infects you. To do this, you will have to maintain personal hygiene and keep your home contamination-free.
Wash your hands thoroughly. If you think you may have come into contact with the virus, you must wash your hands thoroughly to avoid the spread of infection. To wash your hands to avoid contamination, use soap and hot water. Alcohol hand sanitizer is generally considered ineffective against this particular kind of virus. You should wash your hands if[1]:
Avoid cooking for others if you are sick. If you have been infected and are sick, do not handle any food or cook for others in your family. If you do, they are almost certain to get the infection too.
Wash your food before eating or cooking it. Wash all food items such as meats, fruits and vegetables thoroughly before consumption or for use in cooking. This is important as norovirus has the tendency to survive even at temperatures well above 140 degrees Fahrenheit (60 degrees Celsius).[2]
Cook your food thoroughly before eating it. Seafood should be cooked thoroughly before eating it. Quick steaming your food will generally not kill the virus, as it can survive the steaming process. Instead, bake or boil your food at temperatures higher than 140F (60C) if you are concerned about it’s origins.[3]
Use bleach to clean surfaces. Chlorine bleach is an effective cleaning agent that kills norovirus. Increase the concentration or buy a new bottle of chlorine bleach if the bleach you have has been open for more than a month. Bleach becomes less effective the longer it remains open. Before applying bleach to a visible surface, test it somewhere that is not easily seen to make sure that it won’t damage the surface. If the surface is damaged by bleach, you can also use phenolic solutions, such as Pine-Sol, to clean the surface. There are certain concentrations of chlorine bleach you can use for different surfaces.[4]
Rinse surfaces with clean water after using bleach. After cleaning the surfaces, leave the solution to work for 10 to 20 minutes. After the time period elapses, rinse the surface with clean water. After these two steps, close off the area, and leave it like that for one hour.
Clean areas exposed to feces or vomit. For areas exposed to feces or vomit contamination there are special cleaning procedures that you should try to follow. This is because the vomit or feces of a person contaminated with norovirus can easily cause you to become infected. To clean the vomit or feces:
Clean your carpets. If the feces or vomit gets on a carpeted area, there are other steps you can take to make sure that the area is clean and disinfected. To clean the carpeted area:
Disinfect clothing. If any of your clothing or a family member’s clothing has become contaminated, or is suspected of having been contaminated, you should take care when washing the fabric. To clean clothing and linens:
Recognize symptoms. If you think you may have been infected with norovirus, it is helpful to know what symptoms to look for. If you do have the virus, the following steps will help you to deal with the illness while it lasts. Symptoms include[5]:
Understand that while there is no treatment, there are ways to manage symptoms. Unfortunately, there is no specific drug that acts against the virus. However, you can combat the symptoms that the norovirus causes. Remember that the virus is self-limiting, which means that it generally goes away on its own.
Drink lots of fluids. Consuming a lot of water and other fluids will help to keep you hydrated. This can help to keep your fever low and your headaches to a minimum. It is also important to drink water if you have been vomiting or have had diarrhea. When these too symptoms occur, it is very likely that you will become dehydrated.
Consider taking anti-vomiting drugs. Anti-emetic (vomit-preventing) drugs such as ondansetron and domperidone can be given to provide symptomatic relief if you are vomiting frequently.[6]
////////////norovirus, heterocyclic carboxamide, antiviral activity, murine norovirus
Brc1cc(Br)sc1C(=O)Nc2nc3c(F)cc(F)cc3s2
DRUG REGULATORY AFFAIRS INTERNATIONAL

In May, the WHO published a draft guideline which describes the recommendations for ventilation systems used in the manufacture of non-sterile dosage forms. It also contains for the first time a definition for microbial requirements with regard to the zones E and F. Read more about the ventilation sytems recommendations.
In May 2016, the WHO published a draft guideline which describes the recommendations for ventilation systems used in the manufacture of non-sterile dosage forms. From a technical point of view, the guideline is very interesting and includes a detail which may be overlooked: it contains – as first international GMP guideline – a proposal for the definition of microbiological requirements concerning the zones E and F. So far, the approach to extend the zoning via the zones A-D defined in Annex 1 to the zones E and F and thus define microbial limits had only been available in an Aide Memoire…
View original post 84 more words
| Molecular Formula: | C28H31N3O8S |
|---|---|
| Molecular Weight: | 569.62604 g/mol |
| Company | Nimbus Therapeutics LLC |
| Description | Small molecule allosteric inhibitor of acetyl-coenzyme A carboxylase alpha (ACACA; ACC1) and acetyl-coenzyme A carboxylase beta (ACACB; ACC2) |
| Molecular Target | Acetyl-Coenzyme A carboxylase alpha (ACACA) (ACC1) ; Acetyl-Coenzyme A carboxylase beta (ACACB) (ACC2) |
| Mechanism of Action | Acetyl-coenzyme A carboxylase alpha (ACACA) (ACC1) inhibitor; Acetyl-coenzyme A carboxylase beta (ACACB) (ACC2) inhibitor |
| Therapeutic Modality | Small molecule |
Nimbus compounds targeting liver disease in rat models
Data were presented by Geraldine Harriman, from Nimbus Therapeutics, from rat models using acetyl-CoA carboxylase (ACC) inhibitors NDI-010976 (ND-630) and N-654, which improved metabolic syndrome endpoints, decreased liver steatosis, decreased expression of inflammatory markers and improved fibrosis. The hepatotropic ACC inhibitor NDI-010976 had IC50 values of 2 and 7 nM for ACC1 and 2, respectively, EC50 values in HepG2 serum free and 10% serum of 9 and 66 nM, respectively, and 2-fold C2C12 fatty acid oxidation (FAOxn) stimulation at 200 nM. Rat FASyn (synthase), malonyl-CoA (liver) and malonyl-COA (muscle) respective ED50 values were 0.14 mg/kg po, 0.8 and 3 mg/kg. The rat respiratory quotient (RQ) MED was 3 mg/kg po. ADME data showed low multispecies intrinsic clearance (human, mouse, rat, dog, monkey). NDI-010976 was eliminated predominantly as the parent drug. Additionally, P450 inhibition was > 50 microM. In liver and muscle, NDI-010976 modulated key metabolic parameters including a dose-dependent reduction in the formation of the enzymatic product of acetyl coA carboxyloase malonyl coA; the ED50 value was lower in muscle. The drug also decreased FASyn dose dependently and increased fatty acid oxidation in the liver (EC50 = 0.14 mg/kg). In 28-day HS DIO rats, NDI-010976 favorably modulated key plasma and liver lipids, including decreasing liver free fatty acid, plasma triglycerides and plasma cholesterol; this effect was also seen in 37-day ZDF rats

http://www.google.com/patents/WO2013071169A1?cl=en
Example 76: Synthesis of 2-[l-[2-(2-methoxyphenyl)-2-(oxan-4-yloxy)ethyl]-5- methyl-6-(l,3-oxazol-2-yl)-2,4-dioxo-lH,2H,3H,4H-thieno[2,3-d]pyrimidin-3-yl]-2- methylpropanoic acid (1-181).
Synthesis of compound 76.1. Into a 250-mL 3 -necked round-bottom flask, purged and maintained with an inert atmosphere of nitrogen, was placed oxan-4-ol (86 g, 842.05 mmol, 2.01 equiv) and FeCl3 (10 g). This was followed by the addition of 57.2 (63 g, 419.51 mmol, 1.00 equiv) dropwise with stirring at 0 °C. The resulting solution was stirred for 3 h at room temperature. The resulting solution was diluted with 500 mL of H20. The resulting solution was extracted with 3×1000 mL of ethyl acetate and the organic layers combined. The resulting solution was extracted with 3×300 mL of sodium chloride (sat.) and the organic layers combined and dried over anhydrous sodium sulfate. The residue was applied onto a silica gel column with ethyl acetate/petroleum ether (1 : 10). This resulted in 22 g (21%) of 76.1 as a white solid.
Synthesis of compound 76.2. The enantiomers of 76.1 (22g) were resolved by chiral preparative HPLC under the following conditions (Gilson Gx 281): Column: Venusil Chiral OD-
H, 21.1 *25 cm, 5 μιη; mobile phase: hexanes (0.2% TEA) and ethanol (0.2% TEA) (hold at 10% ethanol (0.2%TEA) for 13 min); detector: UV 220/254 nm. 11.4 g (52%) of 76.2 were obtained as a white solid.
Synthesis of compound 76.3. Into a 500-mL 3-necked round-bottom flask, purged and maintained with an inert atmosphere of nitrogen, was placed 70.1 (12 g, 20.49 mmol, 1.00 equiv), tetrahydrofuran (200 mL), 76.2 (6.2 g, 24.57 mmol, 1.20 equiv) and DIAD (6.5 g, 32.18 mmol, 1.57 equiv). This was followed by the addition of a solution of triphenylphosphane (8.4 g, 32.03 mmol, 1.56 equiv) in tetrahydrofuran (100 mL) dropwise with stirring at 0 °C in 60 min. The resulting solution was stirred overnight at room temperature. The resulting mixture was concentrated under vacuum. The residue was applied onto a silica gel column with ethyl acetate/petroleum ether (1 :5). This resulted in 17 g (crude) of 76.3 as a white solid.
Synthesis of compound 76.4. Into a 500-mL 3-necked round-bottom flask, purged and maintained with an inert atmosphere of nitrogen, was placed 76.3 (17 g, crude), toluene (300 mL), Pd(PPh3)4 (1.7 g, 1.47 mmol, 0.07 equiv) and 2-(tributylstannyl)-l,3-oxazole (8.6 g, 24.02 mmol, 1.16 equiv). The resulting solution was stirred overnight at 110 °C. The resulting mixture was concentrated under vacuum. The residue was applied onto a silica gel column with ethyl acetate/petroleum ether (1 : 10). Purification afforded 6 g of 76.4 as a white solid.
Synthesis of compound 1-181. Into a 250-mL 3-necked round-bottom flask, was placed 76.4 (6 g, 7.43 mmol, 1.00 equiv), tetrahydrofuran (100 mL), TBAF (2.3 g, 8.80 mmol,
I .18 equiv). The resulting solution was stirred for 1 h at room temperature. The resulting mixture was concentrated under vacuum. The residue was applied onto a silica gel column with dichloromethane/methanol (50: 1). This resulted in 3.4 g (80%) of Compound 1-181 as a white solid.
Purification: MS (ES): m/z 570 (M+H)+, 592 (M+Na)+.
1H NMR (300 MHz, DMSO- d6): δ 1.22-1.36 (m, 2H), 1.62 (m, 8H), 2.75 (s, 3H), 3.20-3.39 (m, 3H), 3.48-3.58 (m, 2H), 3.80 (s, 3H), 3.85-4.20 (m, 2H), 5.30 (m, 1H), 7.03 (m, 2H), 7.33-7.50 (m, 3H), 8.2 (s, 1H).
Conference: 66th Annual Meeting of the American Association for the Study of Liver Diseases Conference Start Date: 13-Nov-2015
…candidates for minimizing IR injury in liver transplantation.Nimbus compounds targeting liver disease in rat modelsData were presented by Geraldine Harriman, from Nimbus Therapeutics, from rat models using acetyl-CoA carboxylase (ACC) inhibitors NDI-010976 (ND–630) and N-654, which improved metabolic syndrome endpoints, decreased liver steatosis, decreased expression of inflammatory markers and improved fibrosis. The hepatotropic ACC inhibitor NDI-010976 had IC50 values of 2 and 7 nM for ACC1 and 2, respectively…
REFERENCES
| WO-2014182943 |
| WO-2014182945 |
| Patent ID | Date | Patent Title |
|---|---|---|
| US2015203510 | 2015-07-23 | ACC INHIBITORS AND USES THEREOF |
| US2013123231 | 2013-05-16 | ACC INHIBITORS AND USES THEREOF |
/////// ND 630, NDI 010976, ND-630, NDI-010976, NIMBUS, GILEAD, 1434635-54-7
O=C(O)C(C)(C)N4C(=O)c1c(C)c(sc1N(C[C@H](OC2CCOCC2)c3ccccc3OC)C4=O)c5ncco5
Antibodies are used extensively for a wide range of basic research and clinical applications. While an abundant and diverse collection of antibodies to protein antigens have been developed, good monoclonal antibodies to carbohydrates are much less common. Moreover, it can be difficult to determine if a particular antibody has the appropriate specificity, which antibody is best suited for a given application, and where to obtain that antibody. Herein, we provide an overview of the current state of the field, discuss challenges for selecting and using antiglycan antibodies, and summarize deficiencies in the existing repertoire of antiglycan antibodies. This perspective was enabled by collecting information from publications, databases, and commercial entities and assembling it into a single database, referred to as the Database of Anti-Glycan Reagents (DAGR). DAGR is a publicly available, comprehensive resource for anticarbohydrate antibodies, their applications, availability, and quality
Monoclonal antibodies have transformed biomedical research and clinical care. In basic research, these proteins are used widely for a myriad of applications, such as monitoring/detecting expression of biomolecules in tissue samples, activating or antagonizing various biological pathways, and purifying antigens. To illustrate the magnitude and importance of the antibody reagent market, one commercial supplier sells over 50 000 unique monoclonal antibody clones. In a clinical setting, antibodies are used frequently as therapeutic agents and for diagnostic applications. As a result, monoclonal antibodies are a multibillion dollar industry, with antibody therapeutics estimated at greater than $40 billion annually, diagnostics at roughly $8 billion annually, and antibody reagents at $2 billion annually as of 2012
ACS Editors’ Choice – This is an open access article published under an ACS AuthorChoice License, which permits copying and redistribution of the article or any adaptations for non-commercial purposes.
http://pubs.acs.org/doi/full/10.1021/acschembio.6b00244

Jeffrey C. Gildersleeve, Ph.D.
The Gildersleeve group works at the interface of chemistry, glycobiology, and immunology. We use chemical approaches to 1) aid the design and development of cancer and HIV vaccines, 2) identify clinically useful biomarkers, and 3) better understand the roles of carbohydrates in cancer and HIV immunology. To facilitate these studies, we have developed a glycan microarray that allows high-throughput profiling of serum anti-glycan antibody populations.
Link to additional information about Dr. Gildersleeve’s research.
Areas of Expertise
The Gildersleeve group works at the interface of chemistry, glycobiology, and immunology. We use chemical approaches to 1) aid the design and development of cancer and HIV vaccines, 2) identify clinically useful biomarkers, and 3) better understand the roles of carbohydrates in cancer and HIV immunology. To facilitate these studies, we have developed a glycan microarray that allows high-throughput profiling of serum anti-glycan antibody populations. A number of other groups have also developed glycan arrays; our array is unique in that we use multivalent neoglycoproteins as our array components. This format allows us to readily translate array results to other applications and affords novel approaches to vary glycan presentation.
The main focus of our current and future research is to study the roles of anti-glycan antibodies in the development, progression, and treatment of cancer. These projects are shedding new light on how cancer vaccines work and are uncovering new biomarkers for the early detection, diagnosis, and prognosis of cancer. In particular, we are studying immune responses induced by PROSTVAC-VF, a cancer vaccine in Phase III clinical trials for the treatment of advanced prostate cancer. In addition, we are identifying biomarkers for the early detection and prognosis of ovarian and lung cancer. These projects are highly collaborative in nature and are focused on translating basic research from the bench to the clinic. We rely heavily on glycan array technology to study immune responses to carbohydrates, and we continually strive to improve this technology. First, carbohydrate-protein interactions often involve formation of multivalent complexes. Therefore, presentation is a key feature of recognition. We have developed several new approaches to vary carbohydrate presentation on the surface of the array, including methods to vary glycan density and neoglycoprotein density. Second, we use synthetic organic chemistry to obtain a diverse set of tumor-associated carbohydrates and glycopeptides to populate our array.
Collaborations and Carbohydrate Microarray Screening. We are frequently asked to screen lectins, antibodies, and other entities on our array. Although we are not a core facility and do not provide screening services per se, we are happy to collaborate on many projects. Please contact Jeff Gildersleeve for more details.
Scientific Focus Areas:

a small clip
Dr. Eric Sterner, a postdoctoral CRTA Fellow in the Gilderlseeve Lab was presented with a FARE award for his abstract entitled, “Profiling Mutational Significance in Germline-to-Affinity Mature 3F8 Variants” in the NIH-wide FARE 2016 competition. This award is given to abstracts that are deemed outstanding based on scientific merit, originality, experimental design and overall quality and presentation. FARE 2016 is sponsored by the NIH Scientific Directors, the Office of Intramural Training & Education and FelCom. The FARE 2016 Award is a $1000 travel grant to attend and present this work at a scientific meeting within the United States.

Postbaccalaureate Fellow – Cancer Research Training Award (CRTA) at National Cancer Institute (NCI)
https://www.linkedin.com/in/natalie-flanagan-602a98109
June 2015 – Present (1 year 1 month)Frederick, Maryland
September 2014 – May 2015 (9 months)College Park, Maryland
– Ran on section of the Organic Chemistry I laboratory course for two semesters
– Worked with students in a laboratory setting and office hours to help them understand course materials and experimental procedures
– Worked with professors and other TAs to help develop and grade examinations
June 2013 – August 2013 (3 months)Groton, Connecticut
– Used protein crystallization to research ligand binding in a protein kinase system
– Learned a variety of laboratory techniques, including: expression and purification of proteins, and various protein crystallization techniques
– Gained a basic knowledge for how to interpret electron density maps used in three-dimensional protein structure determination
– Presented my research project at an internal poster presentation
2011 – 2015
Activities and Societies: Organic Chemistry Tutor – Primannum Honor Society, Chemistry TA, Phi Beta Kappa, Clinical Volunteer – HSC Pediatric Center
2007 – 2011
//////////Anti-Glycan Antibodies, Gleaned, Community Resource Database
Filed under: ANTIBODIES, Uncategorized Tagged: Anti-Glycan Antibodies, Gleaned, Community Resource Database
![]()