![Image result for MODAFINIL]()
![Modafinil enantiomers.svg]()
MODANAFIL
| Modafinil; 68693-11-8; Provigil; Modiodal; 2-[(diphenylmethyl)sulfinyl]acetamide; Modafinilum [Latin] |
| Molecular Formula: |
C15H15NO2S |
| Molecular Weight: |
273.35 g/mol |
Patent EP0966962 and Patent US2002043207.
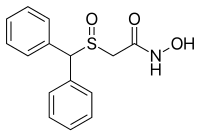
Modafinil (INN,[6] USAN, BAN, JAN) is a wakefulness-promoting agent (or eugeroic) used for treatment of disorders such as narcolepsy, shift work sleep disorder, and excessive daytime sleepiness associated with obstructive sleep apnea.[7] It has also seen widespread off-label use as a purported cognition-enhancing agent. In English-speaking countries it is sold under the brand names Alertec, Modavigil, and Provigil. In the United States modafinil is classified as a schedule IV controlled substance and restricted in availability and usage, due to concerns about possible addiction potential. In most other countries it is a prescription drug but not otherwise legally restricted.
Although the mechanism of action of modafinil was initially unknown, it now appears that the drug acts as a selective, relatively weak, atypical dopamine reuptake inhibitor. However, it appears that other additional mechanisms may also be at play.
![Image result for MODAFINIL]()
History
Modafinil was originally developed in France by neurophysiologist and emeritus experimental medicine professor Michel Jouvet and Lafon Laboratories. Modafinil originated with the late 1970s invention of a series of benzhydryl sulfinyl compounds, including adrafinil, which was first offered as an experimental treatment for narcolepsy in France in 1986. Modafinil is the primary metabolite of adrafinil, lacking the polar -OH group on its terminal amide,[77] and has similar activity to the parent drug but is much more widely used. It has been prescribed in France since 1994 under the name Modiodal, and in the US since 1998 as Provigil.
In 1998, modafinil was approved by the U.S. Food and Drug Administration[78] for the treatment of narcolepsy and in 2003 for shift work sleep disorder and obstructive sleep apnea/hypopnea[79] even though caffeine and amphetamine were shown to be more wakefulness promoting on the Stanford Sleepiness Test Score than modafinil.[80]
It was approved for use in the UK in December 2002. Modafinil is marketed in the US by Cephalon Inc., who originally leased the rights from Lafon, but eventually purchased the company in 2001.
Cephalon began to market the R-enantiomer armodafinil of modafinil in the U.S. in 2007. After protracted patent litigation and negotiations (see below), generic versions of modafinil became available in the U.S. in 2012.
That’s how it went…
![]()
2-benzhydryl-sulfanylacetamide.
Diphenylbromomethane (4,95g = 0.02 moles) and thiourea (1,52g=0.02moles) were refluxed in 20mls water for 30mins. As the synth from Rh’s says, a clear solution must have been formed in 5 mins, but in the end we still had a lot of oil at the bottom (the reasion to blame was old, semidecomposed diphenylbromomethane – when we opened the can, it emitted HBr). We were too lazy to separate the oil , so 2.5g (0.04moles) KOH in 15mls water was added straight and the reflux continued for 30 more mins. A disgusting stench filled the lab.
Thus obtained solution of potassium salt of diphenylmercaptane was cooled to 50-60 C and 1.9g (0.02moles) of chloroacetamide was added thereto. The mixtr was left to its own devices for 2hours – the precipitated oil crystallized. The xtals were filtered, washed thrice w/water, thrice w/ether (removing all benzhydrol). After drying there was obtained 1.9g (37%) of finely divided crystals with mp of 111 C.
With fresh diphenylbromomethane this will give not less than 80% – otherwise I’ll bee a reddish (this is an idiom which I am again unable to translate![Smile smile]() ).
).
Modafinil
Into the solution of 3.6g benzhydrylsulfanylacetamide (0.014moles) in 15mls of GAA there was added 3mls (~0.03moles) 30% hydrogene peroxide. The mixture was left at RT (15 Ñ in our case, better not to heat above) for 20 hrs. Then into the solution there was added 30mls aqua, scratching the walls with a glass rod. After 1 hr the precipitate was filtered, washed w/water twice, then w/ether and dried. Yield – 2,3g (61%), mp – 158-159 C. After some time the mother liquor yielded some more product but we were too lazy to work it up.
PATENT
Patent US2002183552
This is a part of the experimental section:
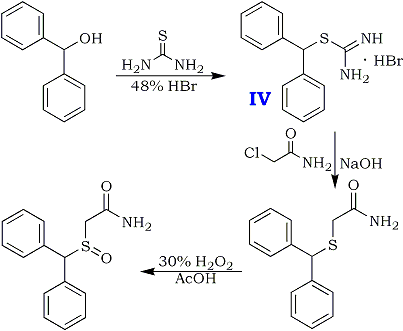
Preparation of isothiouronium Salt (IV).
Diphenylmethanol (130 g, 0.7 mole) and thiourea (65 g, 0.85 mole) are added in 0.5 L reactor charging with water (325 ml). The mixture is heated to 95°C. (an emulsion is obtained) and 48% HBr (260 gr. 3.22 mole, 4.6 equivalents) is then added gradually during 0.5 hour. The mixture is heated under reflux {106-107°C) for 0.5 hour and cooled to 80-85°C. At this temperature, the mixture is seeded with several crystals of the product and the mixture is stirred at that temperature for 0.5 hour and then cooled to 25°C. The colorless crystals are collected by filtration, washed with water (200 ml) yielding about 240 gr. of wet crude isothiouronium salt.
Preparation of diphenylmethylthioacetamide.
A 2 L reactor was charged with diphenylmethylisothiouronium bromide crude wet obtained (240 gr.) and water {700 mL) under nitrogen. The suspension was heated to 60°C and 46% aqueous NaOH solution (98 ml, 1.68 mole, 2.4 eq.) was added. The reaction mixture was heated to 85°C and stirred until all the solid was dissolved. Then, it was cooled to 60°C and chloroacetamide (80 g, O.84 mole, 1.2 eq.) was added in five portions hour at 60-70°C during one hour. The suspension is stirred at 70°C for 4-5 hours. The mixture was filtered while warm and the cake was washed with hot water (250 ml). Diphenylmethylthioacetamide crude wet is obtained [220 gr., HPLC assay: 78%, HPLC purity: 95%, yield: 95% (from diphenylmethanol.)]
20 gr. of the product was recrystallized twice from ethyl acetate, dried in vacuo to give 15 gr. of pure title compound.
Preparation of Modafinil.
A 1.0 L reactor was charged with diphenylmethylthioacetamide crude wet (220 gr.) obtained above and glacial acetic acid (610 mL). The mixture was heated to 40°C and stirred until full dissolution is achieved. 5.8% H2O2 solution (500g, 1.2 eq) was added dropwise during 0.5 hours at 40-45°C. The reaction mixture was stirred at 40-45°C for 4 hours. Then sodium metabisulfite (18.3g) in 610 mL water was added in order to quench the unreacted H2O2 and the suspension was stirred for 0.5 hours. Then the reaction mixture was cooled to 15°C and filtered. The cake was washed with water (610 mL) and dried on air to obtain crude wet Modafinil (205 g). Reslurry in refluxing ethyl acetate, followed by recrystallization from methanol:water (4:1) solution afforded pure Modafinil [125 g, HPLC assay: 99.9%, HPLC purity: 99.9%, yield: 67% (from diphenylmethanol)].![Tongue tongue]()
CLIP
Anti-Narcoleptic Agent Modafinil and Its Sulfone: A Novel
Facile Synthesis and Potential Anti-Epileptic Activity
Nithiananda Chatterjie, James P. Stables, Hsin Wang, and George J. Alexander
Neurochemical Research, Vol. 29, No. 8, August 2004 (© 2004), pp. 1481–1486
![]()
Abstract:
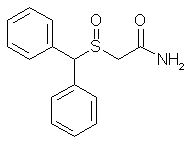
We report a facile procedure to synthesize racemic modafinil (diphenylmethylsulfinylacetamide), which is now being used in pharmacotherapy, and its achiral oxidized derivative (diphenylmethylsulfonyl acetamide). Modafinil is of interest more than for its potential anti-narcoleptic activity. It has also been reported to have neuroprotective properties and may potentially be effective in the enhancement of vigilance and cognitive performance. Finally, it may also protect from subclinical seizures that have been implicated as causative factors in autistic spectrum disorders and other neurodegenerative conditions. This agent can now be synthesized simply and in larger amounts than previously, making it more readily available for testing in various research modalities. The described procedure also lends itself to production of several other amides of potential interest. We are currently in the process of synthesizing and testing several new derivatives in this series. The anticonvulsant properties of modafinil and its sulfone derivative have not previously been extensively described in the literature. It may be of interest to note that the oxidized derivative of modafinil is also nontoxic and almost as effective as an anticonvulsant as the parent.
Experimental
Diphenylmethylthioacetic Acid (3)
Benzhydryl bromide (14.78 gm, 0.059 mole) was dissolved in 75 ml of acetone in a 250-ml round-bottomed flask. To this solution was added dropwise sodium mercaptoacetate (6.59 g, 0.058 mole) in about 60 ml of H2O; the mixture was stirred under N2 for 2 h at room temperature and was thereafter warmed at about 60–70°C for 1 h. The reaction mixture was evaporated to dryness and taken up in CH2Cl2 and saturated aqueous NaHCO3. The organic extract was rejected, and the aqueous phase was treated with acid to pH 2 and chilled. Suction filtration gave the 6.9 g of the acid (3, 46%), mp 125°C. Rf 0.2. Recrystallization from MeOH/H2O gave mp 126–128°C.
Diphenylmethylthioacetamide (4)
Diphenylmethylthioacetic acid (19.5 g, 0.076 mole)
in 114 ml of dry benzene was taken in a 250-ml roundbottomed
flask attached to a reflux condenser, under N2 gas. To this was added thionyl chloride (19.5 ml, 0.097 mole) with a dropping funnel. The mixture was stirred at room temperature with a magnetic stirrer and refluxed for 1 h. Thereafter, the mixture was evaporated under low pressure to give a yellow oil that was taken up in about 100 ml of CH2Cl2 and filtered to yield a clear orange solution. This was chilled in ice water and added slowly to an ice-cold solution of concentrated NH4OH in H2O (40:40 ml). The ensuing mixture was stirred for 1 h and shaken well in a separatory funnel. The organic layer was dried (Na2SO4) and evaporated to dryness to give 14.39 g (54%) of the amide (4), mp 108–109°C (lit2 110°C). Rf 0.8. Recrystallization from CH3OH/H2O gave mp 109–110°C.
Diphenylmethylsulfinylacetamide (modafinil, 1)
Diphenylthioacetamide (3.46 g, 0.013 mole) was taken in glacial acetic acid (14 ml) with stirring; to this was added 1.34 ml of 30% H2O2 with chilling in ice water. The mixture was left in the refrigerator for 4 h and thereafter worked up by treating it with 70 ml of ice-cold water. The precipitated material was filtered under suction and washed with ice-cold water to give 1.5 g of white crystals (43%), mp 159–160°C. Rf 0.6. Recrystallization from hot MeOH gave mp 161–162°C
Diphenylmethylsufonylacetamide (2)
Diphenylmethylthioacetamide (2.5 g, 0.009 mole) (reg. No. 118779-53-6) was dissolved in about 12 ml of glacial acetic acid and 3 ml of 30% H2O2 and set aside overnight (16 h or more). The next day, the mixture was diluted with 100 ml of H2O and set aside to cool in the refrigerator. Upon filtration and drying, 2.1 g (80%) of 2 was obtained as a white powder. Rf 0.89. The melting point of sample after recrystallization from absolute EtOH was 195–197°C.
One aspect of our preparation of modafinil needs further mention. When diphenylmethylthioacetamide (4) is being oxidized by H2O2, care must be taken to keep the reaction mixture cool, and workup should be done in a timely manner. Allowing the reaction to go to 24 h or longer at room temperature results in the formation of the sulfone (2). The paper by Mu et al. (3) does not discuss this possibility. In our hands, the procedure stated therein led to the higher melting sulfone and not the modafinil. Our NMR data for the newly prepared modafinil preparation are in consonance with the data of the patented commercial product. It should be noted that the methylene protons in modafinil are geminally
coupled and appear as a pair of doublets. This is due to the fact that the adjacent sulfoxide moiety is chiral, and therefore the methylene protons adjacent to it wind up being diastereotopic with different chemical shifts and coupling. In the sulfone 2, the methylene protons appear as a singlet due to the fact that the adjacent sulfone moiety is achiral, thus making the two protons equivalent. Modafinil 1 is, however, an equal mixture of enantiomers, as in the reported patent and publication (2,3).
RESULTS
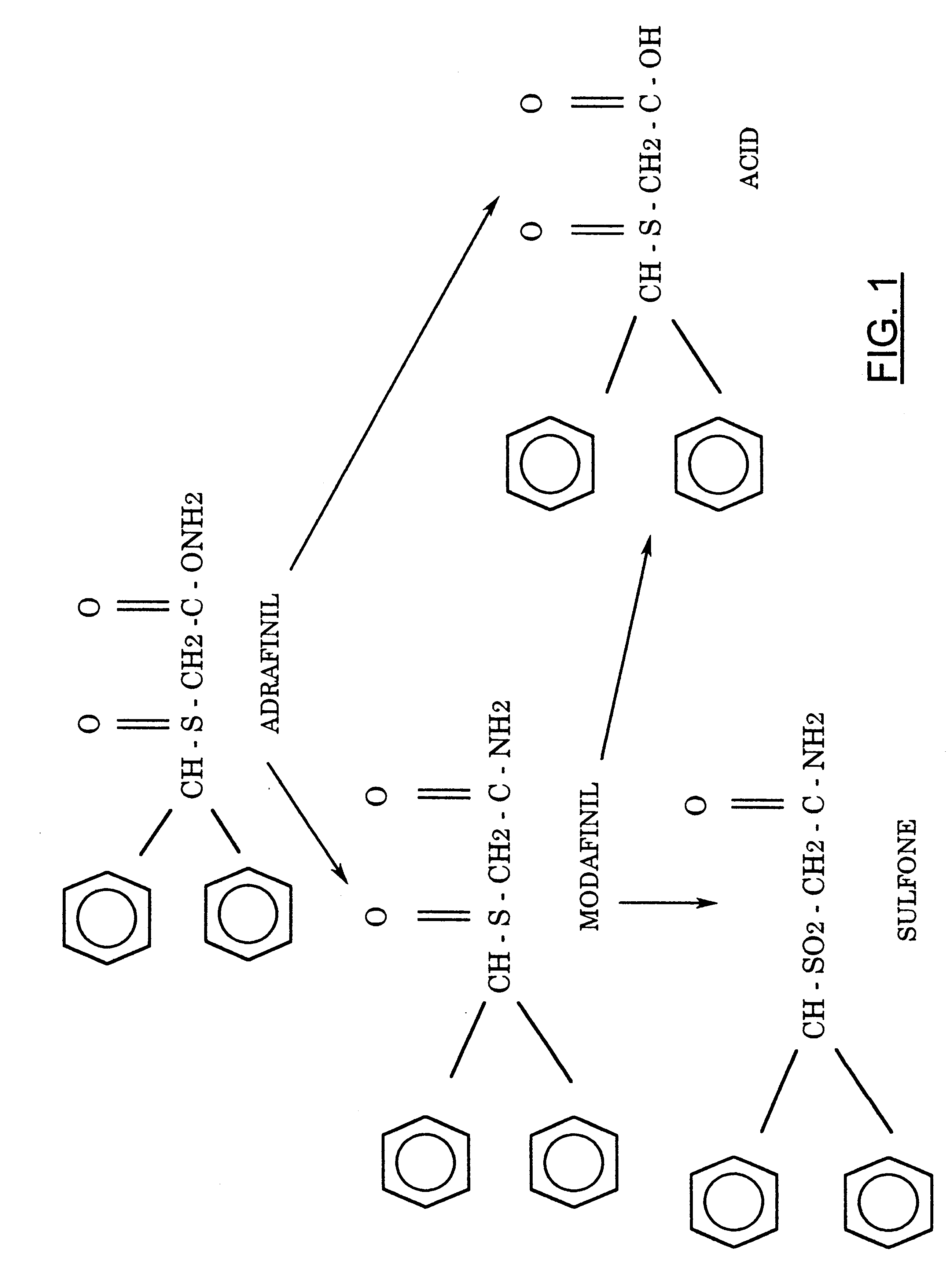
The chemical pathway leading to modafinil may be
represented in Scheme 1.
![]()
see pdf for further information and references,
CLIP
Synthesis and determination of the absolute configuration of the enantiomers of modafinil
Thomas Prisinzanoa, John Podobinskia, Kevin Tidgewella, Min Luoa and Dale Swensonb
Tetrahedron: Asymmetry 15(6), 1053-1058 (2004) (../rhodium/pdf /modafinil.enantiomers.pdf)
DOI:10.1016/j.tetasy.2004.01.039
a Division of Medicinal & Natural Products Chemistry, College of Pharmacy, The University of Iowa, Iowa City, Iowa 52242-1112, USA
b Department of Chemistry, The University of Iowa, Iowa City, Iowa 52242, USA
Abstract
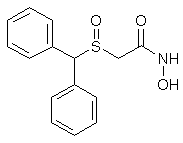
The asymmetric synthesis of both enantiomers of modafinil, a unique CNS stimulant with a reduced abuse liability, is described. This approach effectively prepares modafinil on a multigram scale in several steps from benzhydrol. The described synthetic route has also been used to produce the more water soluble analogue, adrafinil. X-ray crystallographic analysis on (-)-(diphenylmethanesulfinyl)acetic acid has determined the absolute configuration to be R.
Graphical Abstract
![]()
Stereochemistry Abstract
![]()
(S)-(+)-(Diphenylmethanesulfinyl)acetic acid
C15H14O3S
[alpha]D22 + 40.2 (c=1.11, MeOH)
Source of chirality: resolution via diastereomeric salt formation with (R)-(+)-alpha-methylbenzylamine
Absolute configuration: S CLIP
http://www.wmich.edu/cas/experts/docs/2011posters/modafinil.pdf
Narcolepsy is a debilitating neurological disorder which is characterized by chronic sleepiness and is marked to be disorganization of sleep and wake patterns. Every six out of ten thousand people in Western Europe and North America are affected by this disorder. Modafinil (Provigil®) is approved by the Food and Drug Administration for the treatment of narcolepsy. It is commonly used in opposition to Ritalin®, however Ritalin® has an associated dependency issue. Modafinil, a central nervous system stimulant, has reported to have little abuse potential. Modafinil has the ability to act like wake-promoting sympathomimetic agents which includes amphetamine. At relevant pharmacological concentrations modafinil lacks binding ability to receptors for sleep/wake regulation, which includes the ones used for norepinephrine and serotonin. The precise mechanism of action of modafinil is unknown and is presently being researched. Modafinil contains a chiral sulfoxide moiety but is prescribed as a racemate. In collaboration with faculty from the Psychology department at Western Michigan University we were to synthesize modafinil for behavioral studies with animals. Therefore a large scale of pure modafinil was synthesized.
![str0]()
The tetrahedral sulfur atom acts as a chiral center (being surrounded by two dissimilar carbon atoms, an oxygen atom and an electron lone pair (Figure 1). Unlike most analogous trisubstituted amines that undergo umbrella-like inversion at the nitrogen atom, sulfoxides are configurationally stable.
![str1]()
The initial target of this synthesis was to prepare the 2-(diphenylmethylthio)acetamide (1) (Scheme I). The reaction of benzyhydral chloride and thiourea are reacted with potassium iodide, water, heat, 30% sodium hydroxide, 2-chloroacetamide and triethylamine. The procedure required the 2-(diphenylmethylthio) acetamide (1) to be recrystallized to remove any impurities with methanol:water solution 60:40 . After recrystallization (Figure 2) the ¹H NMR spectrum of the synthesized 2-(diphenylmethylthio)acetamide (1) provides evidence that the recrystallization did not purify the compound. In addition recrystallization significantly reduced the percent yield from 78.3-79.2% to 56%. If the compound were pure it would only show peaks at the following locations (ppm): 3.05 (s, 2H), 5.18 (s, 1H), 6.53 (s, 1H), 7.21-7.44(m, 10H).
![str0]()
In preparing (±) modafinil (2) the procedure used acetic acid and hydrogen peroxide to form peracetic acid to react with 2-(diphenylmethylthio)acetamide (1) to form (±) modafinil (2) . The summation of experimentations of Scheme II eventually lead us to use of commercially available peracetic acid to obtain a more pure molecule of (±) modafinil (2). Over oxidation of the sulfone product can be seen if occurs at the peak (ppm):3.7-3.8 in a¹H NMR spectrum of (±) modafinil (2) . ![str1]()
![str2]()
![str0]()
To produce pure 2-(diphenylmethylthio)acetamide (1) elimination of the recrystallization step and 2-(diphenylmethylthio)acetamide (1) was then purified via column chromatography using dichloromethane:ether 80:20 as an eluent with the stationary phase (silica gel). After testing several of the fractions from the column using thin layer chromatography the fractions where able to be identified that contained 2- (diphenylmethylthio)acetamide (1). Once 2-(diphenylmethylthio)acetamide (1) was isolated it was oxidized with peracetic acid. The oxidation process was extended to three hours due to lack of desired product (±) modafinil (Figure 1).
With the procedure we used and modified through experimentation a new procedure was developed that increased the percent yield from 56% to 78.3-79.2%. We encountered a few problems that lead to the removal of the recrystallization step and the use of column chromatography was performed to purify 2-(diphenylmethylthio)acetamide (1) . Over- oxidation could have occurred but would have showed up at 3.7-3.8 (ppm), this did not occur in our experiment. The peak at 1.5 (ppm) is a water peak that was not fully removed during the rotovep procedure. After a precise and confident procedure was perfected then we were able to upscale the reaction and sythesize12gs of pure (±) modafinil.
FROM EROWID………
Benzhydrylsulphinylacetamide (Modafinil)2
Benzhydrylthioacetyl chloride
19.5g (0.076 mol) of benzhydrylthioacetic acid in 114 ml of benzene are placed in a three-necked flask provided with a condenser and a dropping funnel. The mixture is heated and 19 ml of thionyl chloride are added drop by drop. Once the addition is complete, the reflux is continued for about 1 hour, cooling and filtering are carried out and the benzene and the excess thionyl chloride and then evaporated. In this way, a clear orange oil is obtained.
Benzhydrylthioacetamide
35 ml of ammonia in 40 ml of water are introduced into a three-necked flask provided with a condenser and a dropping funnel and the benzhydrylthioacetyl chloride dissolved in about 100 ml of methylene chloride is added drop by drop. Once the addition is complete, the organic phase is washed with a dilute solution of soda and dried over Na2SO4, the solvent is evaporated and the residue is taken up in diisopropyl ether; in this way, the benzhydrylthioacetamide is crystallized. 16.8 g of product (yield 86%) are obtained. M.p. 110°C.
Modafinil (CRL 40,476)
14.39 g (0.056 mol) of benzhydrylthioacetamide are placed in a balloon flask and 60 ml of acetic acid and 5.6 ml of H2O2 (about 110 volumes, 33%) are added. The mixture is left in contact for one night at 40°C. and about 200 ml of water are then added; the CRL 40476 crystallizes. By recrystallization from methanol, 11.2 g of benzhydrylsulphinylacetamide are obtained. Yield: 73%. M.p. 164-66°C.
Novel Synthesis of Modafinil and its sulfone analog3
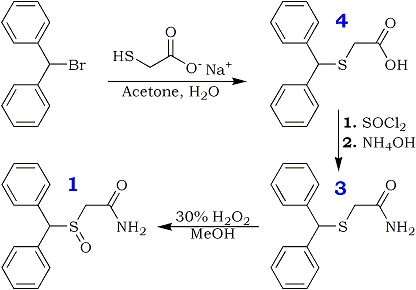
Our interest in synthesis of modified neuroactive compounds has led us to consider Modafinil (1), a stimulant and anti-narcoleptic agent that is finding increasing use in a number of neurological areas. The compound was originally prepared by a rather tedious route described in a procedure patented by L. Lafon2. More recently, its preparation has been reported by Mu et al.4 We believe that this compound has many interesting properties and possible alternative uses in addition to its recognized anti-narcoleptic actions.
Fig 1.
The chemical pathway leading to modafinil
![]()
Not having been able to obtain it from the patent holder, we proceeded to explore alternate synthetic pathways and settled on a convenient synthesis, which permitted us to produce this compound along with a primary derivative, the sulfone (2) in sufficient quantities for whole-animal studies. The current, more facile method starts with benzhydryl bromide and sodium thiolacetate in aqueous acetone, which reacts directly to form diphenylmethylthioacetic acid (3), possibly by an ionic mechanism. This resultant compound can be converted to its acid chloride that, in turn, may be used to acylate ammonia. The ensuing primary amide (4) may be gently oxidized by H2O2 to form the corresponding sulfoxide (Modafinil, 1) and, under more vigorous conditions, the modafinil sulfone (2), whose anticonvulsant and biological properties have not been described extensively in the literature. Additionally, this procedure is also uniquely suitable for large-scale preparation of Modafinil and its congeners.
One aspect of our preparation of modafinil needs further mention. When diphenylmethylthioacetamide (4) is being oxidized by H2O2, care must be taken to keep the reaction mixture cool, and workup should be done in a timely manner. Allowing the reaction to go to 24 h or longer at room temperature results in the formation of the sulfone (2). The paper by Mu et al.4 does not discuss this possibility. In our hands, the procedure stated therein led to the higher melting sulfone and not the modafinil. Our NMR data for the newly prepared modafinil preparation are in consonance with the data of the patented commercial product. It should be noted that the methylene protons in modafinil are geminally coupled and appear as a pair of doublets. This is due to the fact that the adjacent sulfoxide moiety is chiral, and therefore the methylene protons adjacent to it wind up being diastereotopic with different chemical shifts and coupling. In the sulfone 2, the methylene protons appear as a singlet due to the fact that the adjacent sulfone moiety is achiral, thus making the two protons equivalent. Modafinil 1 is, however, an equal mixture of enantiomers, as in the reported patent and publication2,4.
Experimental
The new compounds were prepared according to modified procedures published in the patent literature. Starting materials and solvents were obtained commercially from Fluka and/or Aldrich Chemical Corp. Thin layer chromatography (TLC) was performed on silica gel plates. Solvent system was EtOAc:MeOH:NH4OH, 100:10:3 by volume. Melting points are uncorrected.
Diphenylmethylthioacetic Acid (3)
Benzhydryl bromide (14.78 gm, 0.059 mole) was dissolved in 75 ml of acetone in a 250-ml round-bottomed flask. To this solution was added dropwise sodium mercaptoacetate (6.59 g, 0.058 mole) in about 60 ml of H2O; the mixture was stirred under N2 for 2 h at room temperature and was thereafter warmed at about 60–70°C for 1 h. The reaction mixture was evaporated to dryness and taken up in CH2Cl2 and saturated aqueous NaHCO3. The organic extract was rejected, and the aqueous phase was treated with acid to pH 2 and chilled. Suction filtration gave the 6.9 g of the acid (3, 46%), mp 125°C. Rf 0.2. Recrystallization from MeOH/H2O gave mp 126–128°C.
Diphenylmethylthioacetamide (4)
Diphenylmethylthioacetic acid 3 (19.5 g, 0.076 mole) in 114 ml of dry benzene was taken in a 250-ml roundbottomed flask attached to a reflux condenser, under N2 gas. To this was added thionyl chloride (19.5 ml, 0.097 mole) with a dropping funnel. The mixture was stirred at room temperature with a magnetic stirrer and refluxed for 1 h. Thereafter, the mixture was evaporated under low pressure to give a yellow oil that was taken up in about 100 ml of CH2Cl2 and filtered to yield a clear orange solution. This was chilled in ice water and added slowly to an ice-cold solution of concentrated NH4OH in H2O (40:40 ml). The ensuing mixture was stirred for 1 h and shaken well in a separatory funnel. The organic layer was dried (Na2SO4) and evaporated to dryness to give 14.39 g (54%) of the amide (4), mp 108–109°C (lit4 110°C). Rf 0.8. Recrystallization from CH3OH/H2O gave mp 109–110°C.
Diphenylmethylsulfinylacetamide (Modafinil, 1)
Diphenylmethylthioacetamide 4 (3.46 g, 0.013 mole) was taken in glacial acetic acid (14 ml) with stirring; to this was added 1.34 ml of 30% H2O2 with chilling in ice water. The mixture was left in the refrigerator for 4 h and thereafter worked up by treating it with 70 ml of ice-cold water. The precipitated material was filtered under suction and washed with ice-cold water to give 1.5 g of white crystals (43%), mp 159–160°C. Rf 0.6. Recrystallization from hot MeOH gave mp 161–162°C
Diphenylmethylsulfonylacetamide (2)
Diphenylmethylthioacetamide (2.5 g, 0.009 mole) (CAS No. 118779-53-6) was dissolved in about 12 ml of glacial acetic acid and 3 ml of 30% H2O2 and set aside overnight (16 h or more). The next day, the mixture was diluted with 100 ml of H2O and set aside to cool in the refrigerator. Upon filtration and drying, 2.1 g (80%) of 2 was obtained as a white powder. Rf 0.89. The melting point of sample after recrystallization from absolute EtOH was 195–197°C.
High-yield Synthesis of Modafinil from Benzhydrol5
A recent patent5 describes a very easy two-step route to the Modafinil precursor diphenylmethanethioacetamide from benzhydrol (diphenylmethanol) in 90% yield and with 95% purity. A 200g batch is made in a 2000 mL vessel using water as reaction medium and ethyl acetate for recrystallization of the product.
Diphenylmethylbromide is prepared in situ from benzhydrol and react it with thiourea in a one-pot reaction to form the corresponding isothiouronium salt. The crude salt is then reacted with chloroacetamide (by generating the thiolate cation in situ), and after filtration and washing, diphenylmethylthioacetamide is isolated in excellent yield and good purity. After oxidation of the thioacetamide with hydrogen peroxide, followed by recrystallization, the overall yield of Modafinil is 67% from the benzhydrol.
(Chimimanie’s Voice:) The synthesis works just as great without the nitrogen inert atmosphere (most patents do not use it at all), step two is only a hydrolysis of the thiouronium salt to the thiolate. You just have to put the salt, NaOH and heat till you got a homogenous solution, with no more solid material floating around. The following chloroacetamide SN2 reaction is a breeze too. Sometime a blue solution can bee obtained, it is nothing to worry about. In the final step, you just have to filter off the solid which did not dissolve when the crude thioacetamide is put in the GAA/H2O2, bee4 crashing the soluble one with water.
Do not forget to slurry the modafinil in EtOAc and then recrystallize it from aqueous MeOH, as the crystalline shape of modafinil is important for the kinetic and quality of effects, at least according to the patents EP0966962 and US2002043207.
Experimental
Preparation of isothiouronium Salt (IV)
Diphenylmethanol (130 g, 0.7 mole) and thiourea (65 g, 0.85 mole) are added in 0.5 L reactor charging with water (325 ml). The mixture is heated to 95°C. (an emulsion is obtained) and 48% HBr (260 gr. 3.22 mole, 4.6 equivalents) is then added gradually during 0.5 hour. The mixture is heated under reflux (106-107°C) for 0.5 hour and cooled to 80-85°C. At this temperature, the mixture is seeded with several crystals of the product and the mixture is stirred at that temperature for 0.5 hour and then cooled to 25°C. The colorless crystals are collected by filtration, washed with water (200 ml) yielding about 240 gr. of wet crude isothiouronium salt.
(Antoncho’s Voice:) Assholium successfully made Modafinil by this method, but there turned out to be a mistake in the original patent text – In the preparation of IV, the quantity of HBr stated here is excessive and leads to complete hydrolysis of the initially formed isothiouronium salt. The acid should bee added until the reaction mixture turns completely clear (about half as much as the patent says) – a sort of titration. Further addition will result in precipitation of heavy stinky oil, benzhydrylmethanethiol.
Preparation of diphenylmethylthioacetamide
A 2 L reactor was charged with diphenylmethylisothiouronium bromide crude wet obtained (240 gr.) and water (700 mL) under nitrogen. The suspension was heated to 60°C and 46% aqueous NaOH solution (98 ml, 1.68 mole, 2.4 eq.) was added. The reaction mixture was heated to 85°C and stirred until all the solid was dissolved. Then, it was cooled to 60°C and chloroacetamide (80 g, 0.84 mole, 1.2 eq.) was added in five portions hour at 60-70°C during one hour. The suspension is stirred at 70°C for 4-5 hours. The mixture was filtered while warm and the cake was washed with hot water (250 ml). Diphenylmethylthioacetamide crude wet is obtained [220 gr., HPLC assay: 78%, HPLC purity: 95%, yield: 95% from diphenylmethanol]. 20g of the product was recrystallized twice from ethyl acetate, dried in vacuo to give 15g of pure title compound.
Preparation of Modafinil
A 1.0 L reactor was charged with diphenylmethylthioacetamide crude wet (220 gr.) obtained above and glacial acetic acid (610 mL). The mixture was heated to 40°C and stirred until full dissolution is achieved. 5.8% H2O2 solution (500g, 1.2 eq) was added dropwise during 0.5 hours at 40-45°C. The reaction mixture was stirred at 40-45°C for 4 hours. Then sodium metabisulfite (18.3g) in 610 mL water was added in order to quench the unreacted H2O2 and the suspension was stirred for 0.5 hours. Then the reaction mixture was cooled to 15°C and filtered. The cake was washed with water (610 mL) and dried on air to obtain crude wet Modafinil (205 g). Reslurry in refluxing ethyl acetate, followed by recrystallization from methanol:water (4:1) solution afforded pure Modafinil [125 g, HPLC assay: 99.9%, HPLC purity: 99.9%, yield: 67% (from diphenylmethanol)].
References
- US Pat 4,066,686
- L. Lafon, US Pat 4,177,290 (1979); L. Lafon, Eur. Pat. 283,362 (1988)
- Nithiananda Chatterjie, James P. Stables, Hsin Wang, and George J. Alexander, Anti-Narcoleptic Agent Modafinil and Its Sulfone: A Novel Facile Synthesis and Potential Anti-Epileptic Activity, Neurochemical Research, 29(8), 1481–1486 (2004)
- Mu, B., Lei, G., He, X., and Du, X., Synthesis of central stimulant modafinil. Zhongguo Yaowu Huaxue Zazhi, 9(2), 132–134 (1999)
- US Pat. 6,649,796 (2002)
![]()
Green Chem., 2017, Advance Article
DOI: 10.1039/C6GC02623K, Communication
Shivam Maurya, Dhiraj Yadav, Kemant Pratap, Atul Kumar
We developed a post-sulfoxidation protocol for the synthesis of Modafinil that exhibits improved sustainability credentials, utilizing the recyclable heterogeneous catalyst Nafion-H.
Efficient atom and step economic (EASE) synthesis of the “smart drug” Modafinil
*Corresponding authors
aMedicinal & Process Chemistry Division, CSIR-Central Drug Research Institute, Sector 10, Jankipuram Extension, Sitapur Road, P.O. Box 173, Lucknow 226031, India
E-mail: dratulsax@gmail.com,
atul_kumar@cdri.res.in
bAcademy of Scientific and Innovative Research, New Delhi 110001, India
Green Chem., 2017, Advance Article
DOI: 10.1039/C6GC02623K
![]()
Professor, Academy of Scientific and Innovative Research (AcSIR)/ Senior Principal Scientist at CSIR-CDRI
Lucknow, Uttar Pradesh, India
Modafinil (2-[(diphenylmethyl)sulfinyl]acetamide, MOD) is a key psychostimulant drug used for the treatment of narcolepsy and other sleep disorders that has a very low addiction liability. Recently, MOD has been clinically investigated for the treatment of cocaine addiction and used by astronauts in long-term space missions. We have developed a synthetic strategy for “smart drug” Modafinil. An efficient atom and step economic (EASE) synthesis has been carried out by the direct reaction of benzhydrol and 2-mercaptoacetamide using the recyclable heterogeneous catalyst Nafion-H along with post-sulfoxidation. This protocol exhibits improved sustainability credentials. We have also developed a superior pre-sulfoxidation approach for the synthesis of Modafinil.
![]()
Modafinil Physical State – White solid; M.p. 158-159ºC,
IR (KBr): 3383, 3314, 3256, 1690, 1 1616, 1494, 1376, 1027, 702 cm-1;
H NMR (CDCl3) δ(ppm): 3.14(d, J=14.3 Hz, 1H); 3.48(d, J=14.3 Hz, 1H); 5.24(s, 1H); 5.88(br s, 1H); 7.09(br s, 1H); 7.29-7.43(m, 7H); 7.43-7.51(m, 3H);
13C NMR (CDCl3) δ(ppm): 52.00, 71.61, 128.80, 128.98, 129.10, 129.58, 129.62, 134.30, 134.74, + 166.46; Molecular formula C15H15NO2S;
ESI-MS (m/z): 274.1 (M+H) .
Dr. Atul Kumar
Senior Principal Scientist
![]()
![str0]()
////////////
Filed under:
Uncategorized Tagged:
MODANAFIL ![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()




























 Nidhi Adlaka & Neha Munjal are developing a bioprocess for butanediol. Over the next few decades, chemical routes of manufacture will gradually be replaced by more environment friendly biological methods.
Nidhi Adlaka & Neha Munjal are developing a bioprocess for butanediol. Over the next few decades, chemical routes of manufacture will gradually be replaced by more environment friendly biological methods.